Abstract
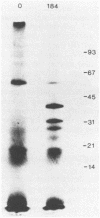
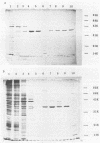
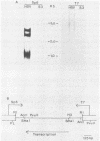
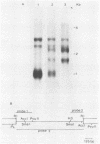
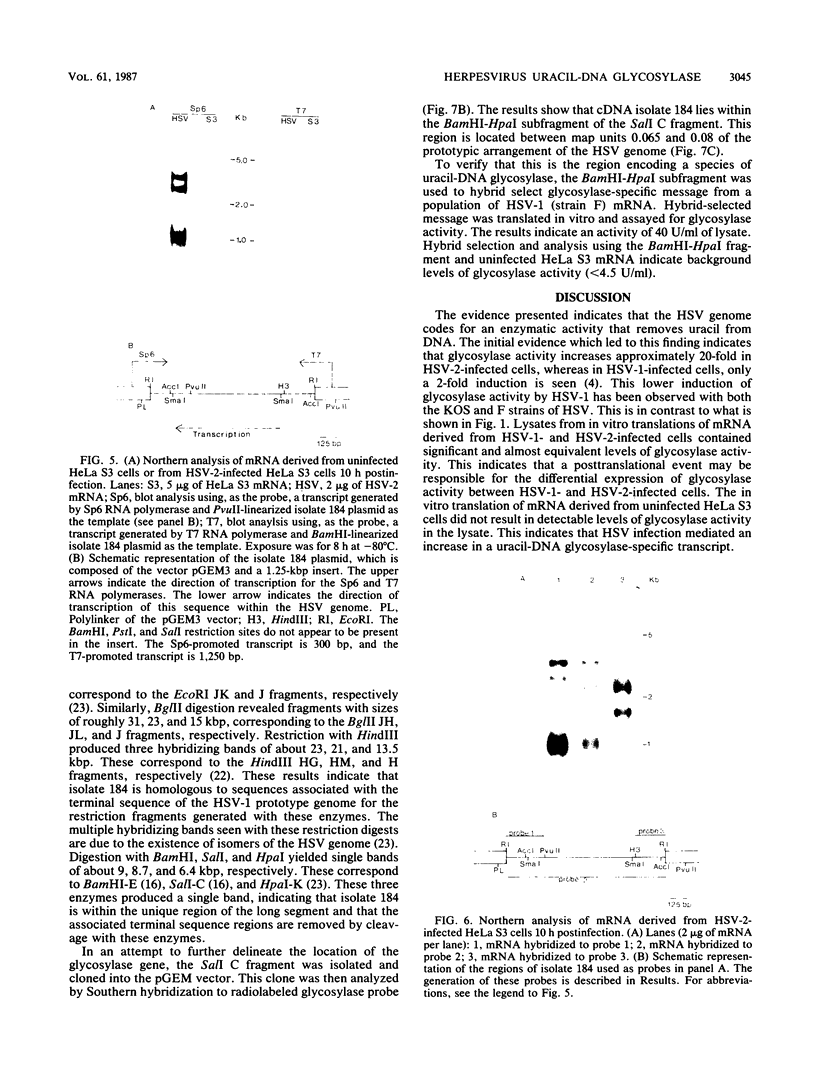
Activity of the DNA repair enzyme uracil-DNA glycosylase has been shown to increase in herpes simplex virus type 2 (HSV-2)-infected cells. When mRNA derived from either HSV-1- or HSV-2-infected HeLa S3 cells was translated in an in vitro translation system, significant uracil-DNA glycosylase activity could be detected in the lysate. This activity was specific for the removal of uracil from DNA. Lysates from in vitro translation of mRNA derived from uninfected HeLa cells did not contain measurable glycosylase activity. A cDNA library was constructed with mRNA derived from HSV-2-infected cells 10 h postinfection. Pooled isolates from this library were used in hybrid-arrest and in vitro translation reactions to isolate a uracil-DNA glycosylase-specific cDNA. In vitro translation of hybrid-selected RNA, by using this cDNA, produced glycosylase activity in the lysate. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of radiolabeled products from this translation reaction showed a protein component with a molecular weight of 39,000. This is consistent with the molecular weight determinations of the purified glycosylase enzyme derived from either uninfected or HSV-infected HeLa cells. Northern (RNA blot) analysis of HSV-derived RNA, by using the glycosylase cDNA as a probe, revealed five overlapping transcripts of 3.4, 2.8, 2.4, 1.7, and 1.0 kilobases. Southern analysis indicated that the DNA sequence encoding the HSV-specific uracil-DNA glycosylase was located between 0.065 and 0.08 map units on the prototypic arrangement of the HSV genome.
Full text
PDF

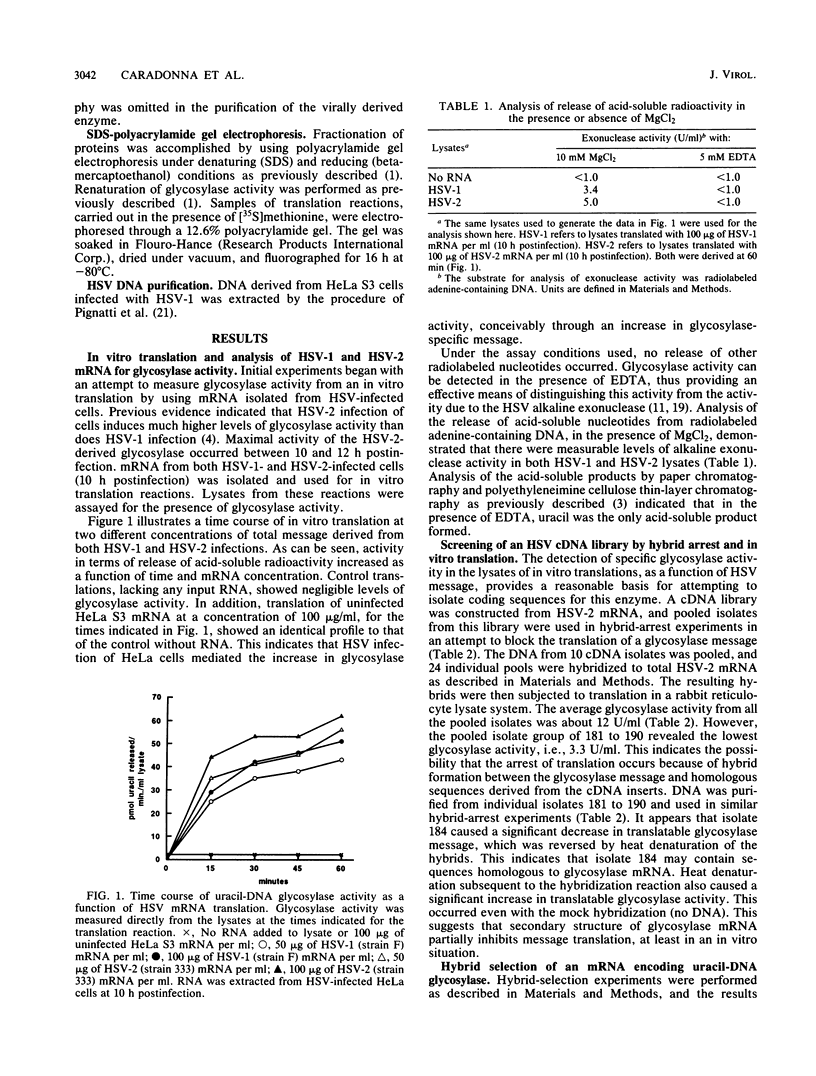


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
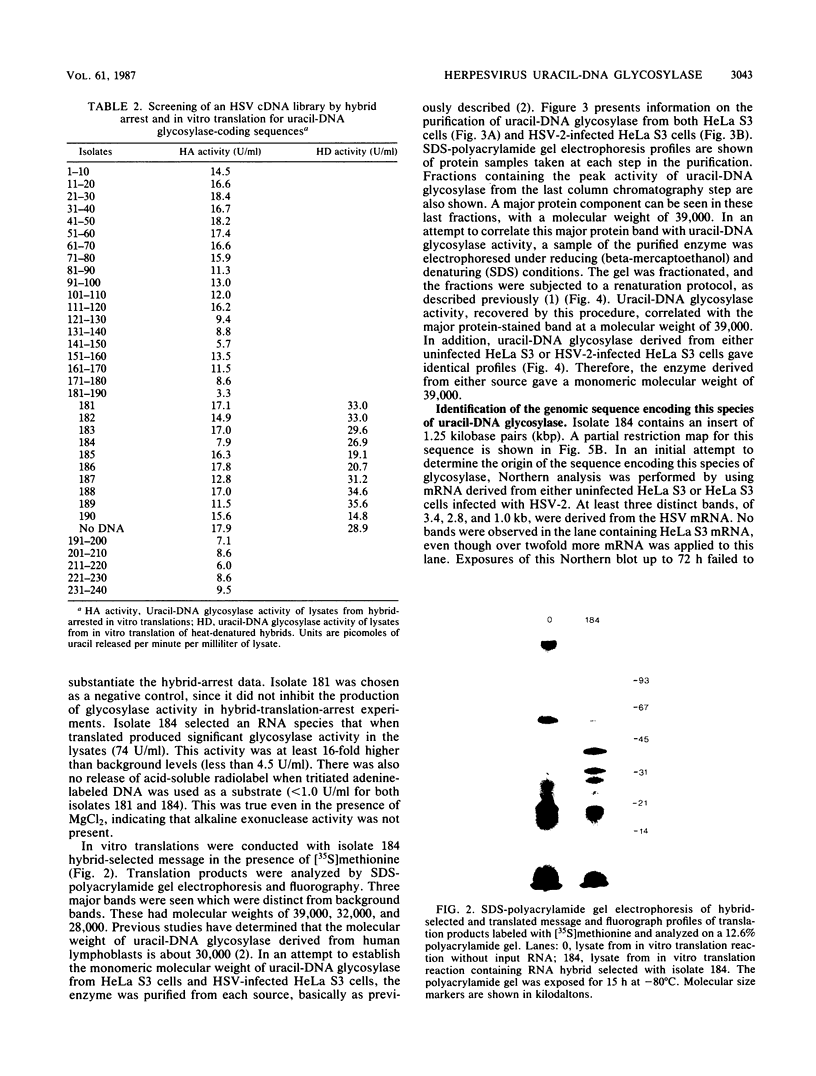
- Caradonna S. J., Adamkiewicz D. M. Purification and properties of the deoxyuridine triphosphate nucleotidohydrolase enzyme derived from HeLa S3 cells. Comparison to a distinct dUTP nucleotidohydrolase induced in herpes simplex virus-infected HeLa S3 cells. J Biol Chem. 1984 May 10;259(9):5459–5464. [PubMed] [Google Scholar]
- Caradonna S. J., Cheng Y. C. DNA glycosylases. Mol Cell Biochem. 1982 Jul 7;46(1):49–63. doi: 10.1007/BF00215581. [DOI] [PubMed] [Google Scholar]
- Caradonna S. J., Cheng Y. C. Induction of uracil-DNA glycosylase and dUTP nucleotidohydrolase activity in herpes simplex virus-infected human cells. J Biol Chem. 1981 Oct 10;256(19):9834–9837. [PubMed] [Google Scholar]
- Caradonna S. J., Cheng Y. C. The role of deoxyuridine triphosphate nucleotidohydrolase, uracil-DNA glycosylase, and DNA polymerase alpha in the metabolism of FUdR in human tumor cells. Mol Pharmacol. 1980 Nov;18(3):513–520. [PubMed] [Google Scholar]
- Caradonna S. J., Cheng Y. C. Uracil DNA-glycosylase. Purification and properties of this enzyme isolated from blast cells of acute myelocytic leukemia patients. J Biol Chem. 1980 Mar 25;255(6):2293–2300. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
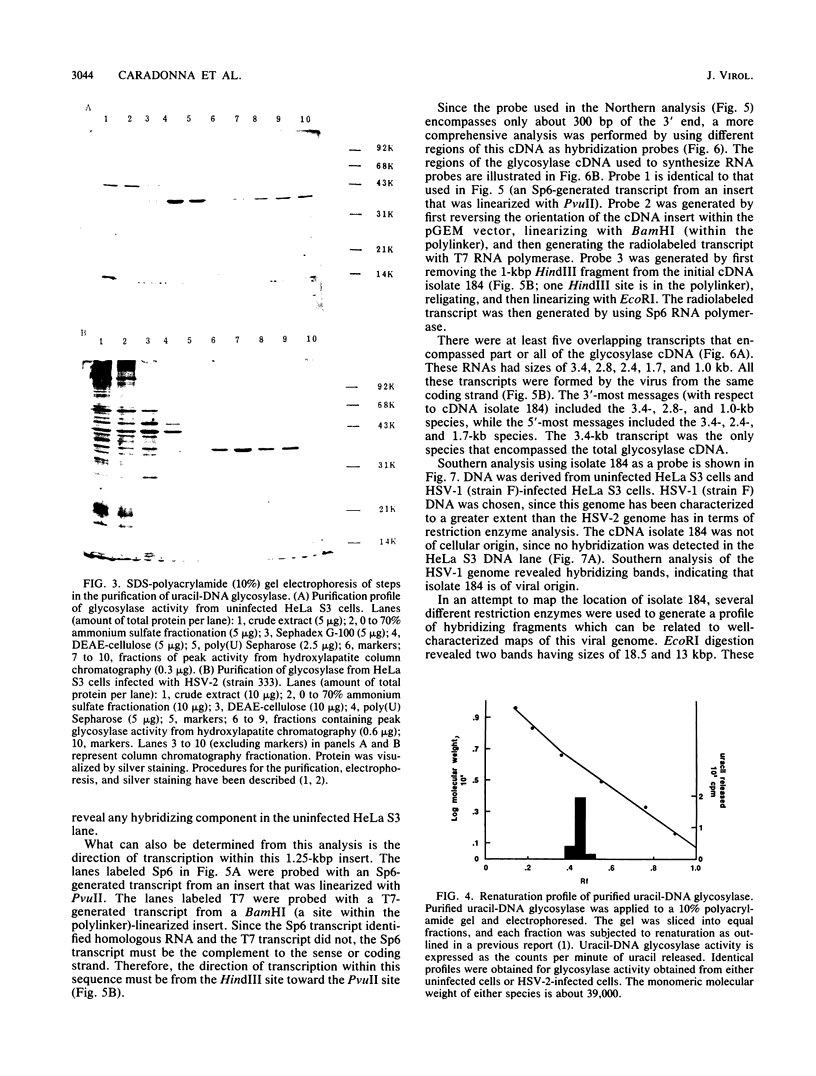
- Cone R., Duncan J., Hamilton L., Friedberg E. C. Partial purification and characterization of a uracil DNA N-glycosidase from Bacillus subtilis. Biochemistry. 1977 Jul 12;16(14):3194–3201. doi: 10.1021/bi00633a024. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Banks L., Powell K. L., Cohen G., Eisenberg R., Wagner E. K. High-resolution characterization of herpes simplex virus type 1 transcripts encoding alkaline exonuclease and a 50,000-dalton protein tentatively identified as a capsid protein. J Virol. 1983 Dec;48(3):591–603. doi: 10.1128/jvi.48.3.591-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Locker H., Frenkel N. BamI, KpnI, and SalI restriction enzyme maps of the DNAs of herpes simplex virus strains Justin and F: occurrence of heterogeneities in defined regions of the viral DNA. J Virol. 1979 Nov;32(2):429–441. doi: 10.1128/jvi.32.2.429-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Frame M. C. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 1986 Apr 25;14(8):3435–3448. doi: 10.1093/nar/14.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti P. F., Cassai E., Meneguzzi G., Chenciner N., Milanesi G. Herpes simplex virus DNA isolation from infected cells with a novel procedure. Virology. 1979 Feb;93(1):260–264. doi: 10.1016/0042-6822(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Poffenberger K. L., Roizman B. A noninverting genome of a viable herpes simplex virus 1: presence of head-to-tail linkages in packaged genomes and requirements for circularization after infection. J Virol. 1985 Feb;53(2):587–595. doi: 10.1128/jvi.53.2.587-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. The organization of the herpes simplex virus genomes. Annu Rev Genet. 1979;13:25–57. doi: 10.1146/annurev.ge.13.120179.000325. [DOI] [PubMed] [Google Scholar]