Abstract
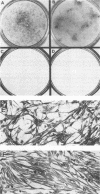
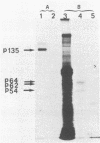
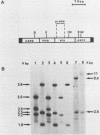
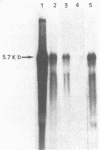
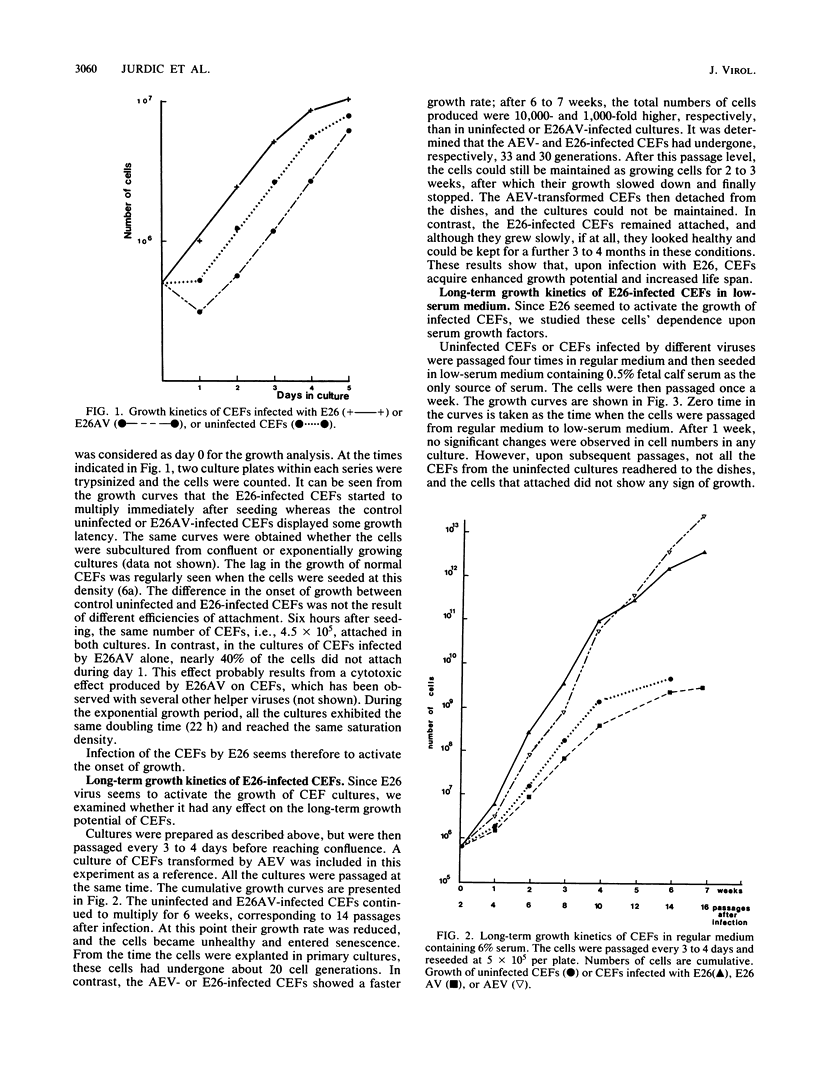
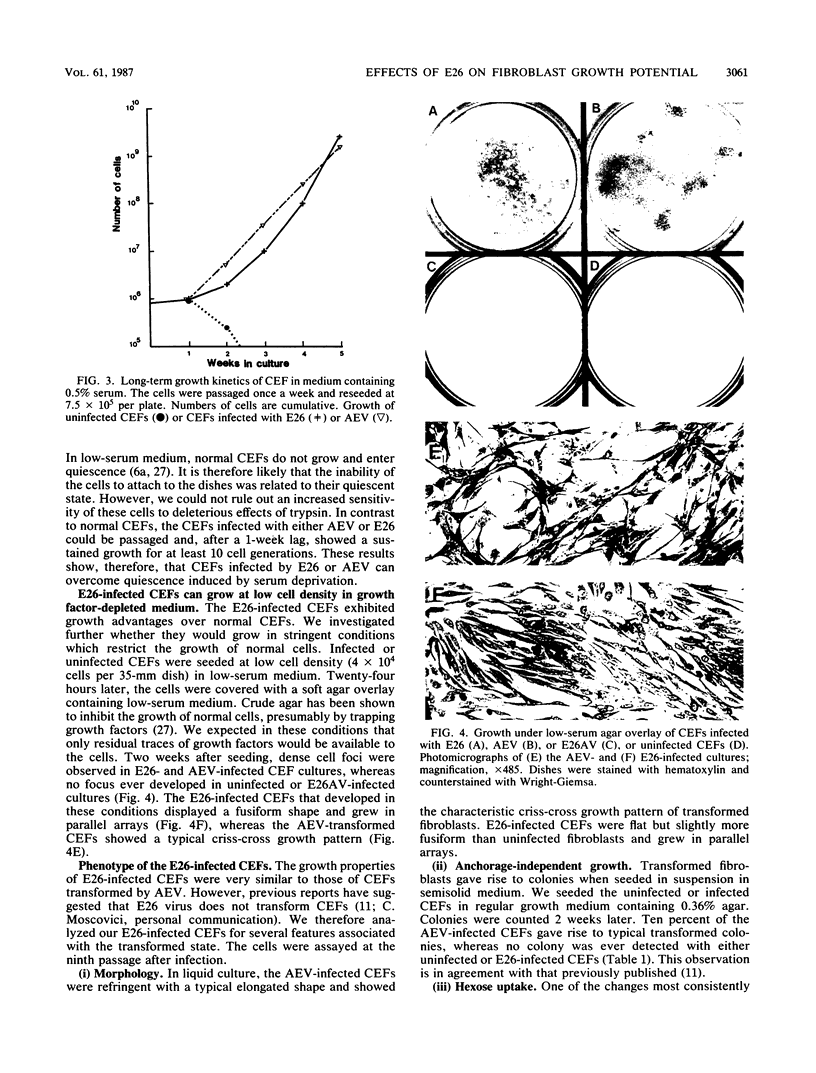
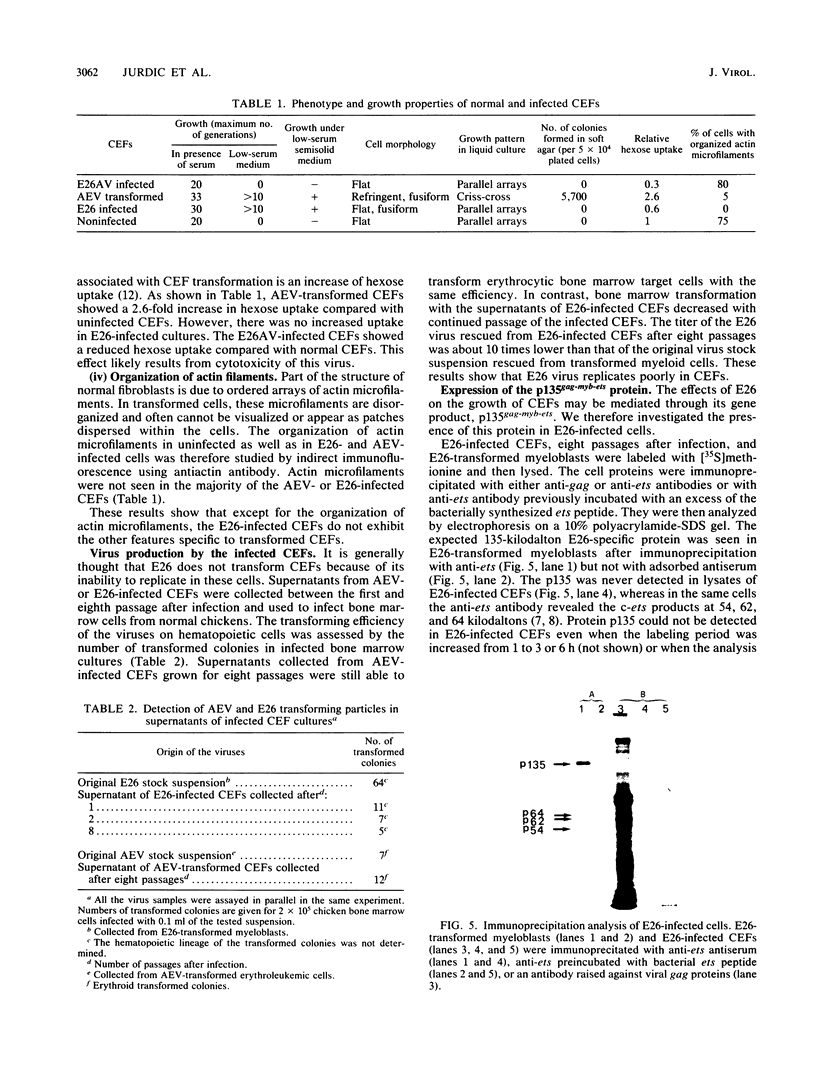
We have investigated the effect of E26, an avian leukemia retrovirus, on the growth properties of chicken embryo fibroblasts (CEFs). E26-infected CEFs were not transformed, according to several transformation parameters, but exhibited an activated growth in vitro. They started to grow without latency in serum-supplemented medium, maintained long-term growth in regular or low-serum medium, and could grow when seeded at low cell density in low-serum medium. We compared the integration and the level of expression of the proviral DNA in E26-infected CEFs and E26-transformed hematopoietic cells. An average of two provirus copies were found in each kind of cells. However, whereas high contents of both viral mRNA and E26-specific protein products were found in transformed hematopoietic cells, we detected only low amounts of viral mRNA and no E26 protein in infected CEFs. These data show that the level of expression of the E26 provirus is lower in CEFs than in hematopoietic cells. They suggest that transformation efficiency of the virus depends on its level of expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Bister K., Nunn M., Moscovici C., Perbal B., Baluda M., Duesberg P. H. Acute leukemia viruses E26 and avian myeloblastosis virus have related transformation-specific RNA sequences but different genetic structures, gene products, and oncogenic properties. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3677–3681. doi: 10.1073/pnas.79.12.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge W. H., Moscovici C. Colony formation by chicken hematopoietic cells and virus-induced myeloblasts. J Cell Physiol. 1973 Jun;81(3):371–386. doi: 10.1002/jcp.1040810310. [DOI] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Benchaibi M., Xiao J. H., Ghysdael J., Samarut J. Expression of the v-erbA oncogene in chicken embryo fibroblasts stimulates their proliferation in vitro and enhances tumor growth in vivo. Cell. 1987 Jun 5;49(5):687–697. doi: 10.1016/0092-8674(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Boulukos K., Leprince D., Dernis D., Lagrou C., Stehelin D. Identification in chicken macrophages of a set of proteins related to, but distinct from, the chicken cellular c-ets-encoded protein p54c-ets. EMBO J. 1986 Sep;5(9):2251–2256. doi: 10.1002/j.1460-2075.1986.tb04492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Dernis D., Leprince D., Stehelin D. Identification and preferential expression in thymic and bursal lymphocytes of a c-ets oncogene-encoded Mr 54,000 cytoplasmic protein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1714–1718. doi: 10.1073/pnas.83.6.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Samarut J., Gazzolo L., Moscovici M. G. Myeloid and erythroid neoplastic responses to avian defective leukemia viruses in chickens and in quail. Virology. 1981 Sep;113(2):765–768. doi: 10.1016/0042-6822(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Nunn M., Weiher H., Bullock P., Duesberg P. Avian erythroblastosis virus E26: nucleotide sequence of the tripartite onc gene and of the LTR, and analysis of the cellular prototype of the viral ets sequence. Virology. 1984 Dec;139(2):330–339. doi: 10.1016/0042-6822(84)90378-7. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Samarut J., Gazzolo L. Target cells infected by avian erythroblastosis virus differentiate and become transformed. Cell. 1982 Apr;28(4):921–929. doi: 10.1016/0092-8674(82)90071-x. [DOI] [PubMed] [Google Scholar]
- Senear A. W., Lewis J. B. Morphological transformation of established rodent cell lines by high-level expression of the adenovirus type 2 E1a gene. Mol Cell Biol. 1986 Apr;6(4):1253–1260. doi: 10.1128/mcb.6.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Symonds G., Quintrell N., Stubblefield E., Bishop J. M. Dispersed chromosomal localization of the proto-oncogenes transduced into the genome of Mill Hill 2 or E26 leukemia virus. J Virol. 1986 Jul;59(1):172–175. doi: 10.1128/jvi.59.1.172-175.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. VI. Differential multiplication of uninfected and of converted cells in response to insulin. J Cell Physiol. 1967 Jun;69(3):377–384. doi: 10.1002/jcp.1040690314. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J. Immortalization of primary mouse embryo fibroblasts with SV40 virions, viral DNA, and a subgenomic DNA fragment in a quantitative assay. Virology. 1984 Sep;137(2):414–421. doi: 10.1016/0042-6822(84)90234-4. [DOI] [PubMed] [Google Scholar]
- Vogt M., Haggblom C., Swift S., Haas M. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J Virol. 1985 Jul;55(1):184–192. doi: 10.1128/jvi.55.1.184-192.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]