Abstract
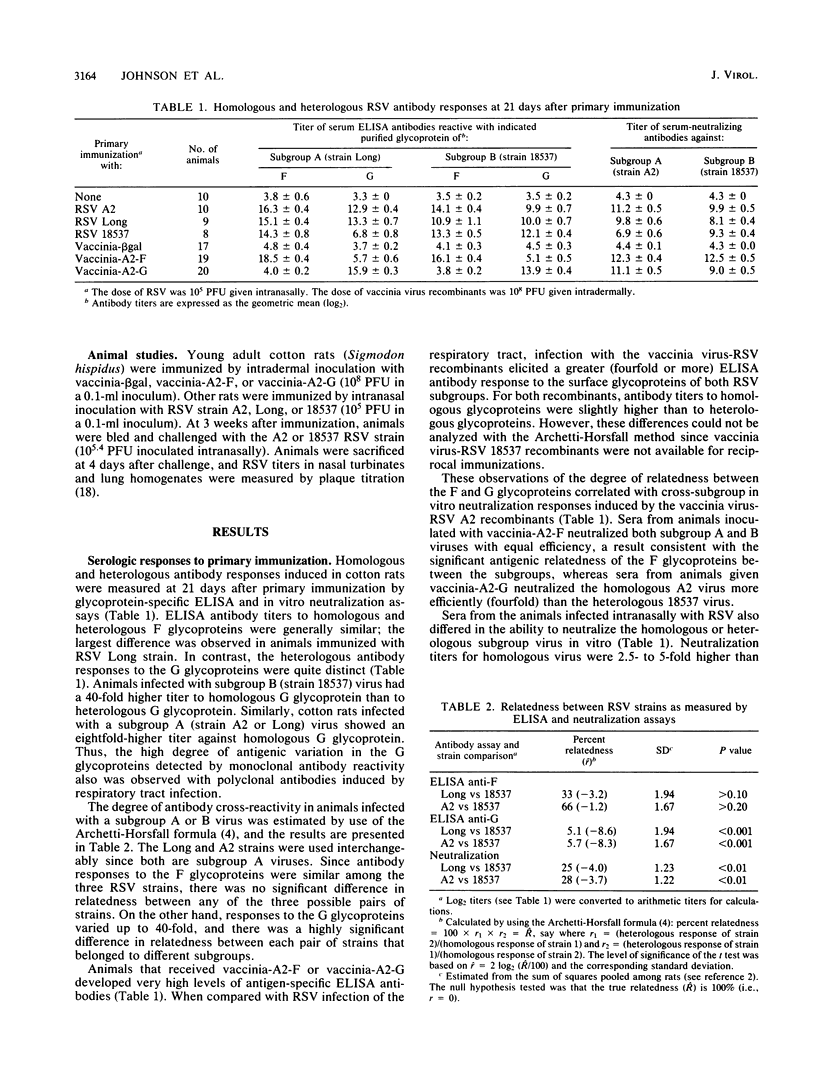
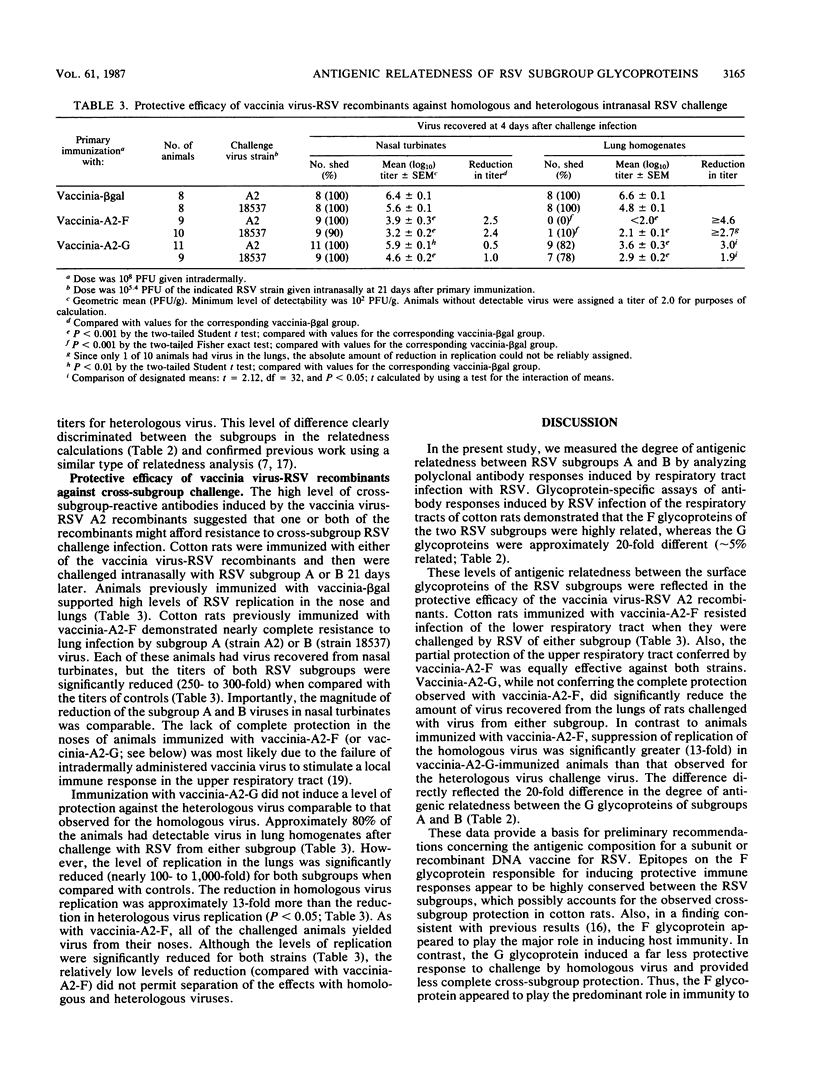
The degree of antigenic relatedness between human respiratory syncytial virus (RSV) subgroups A and B was estimated from antibody responses induced in cotton rats by respiratory tract infection with RSV. Glycoprotein-specific enzyme-linked immunosorbent assays of antibody responses induced by RSV infection demonstrated that the F glycoproteins of subgroups A and B were antigenically closely related (relatedness, R approximately 50%), whereas the G glycoproteins were only distantly related (R approximately 5%). Intermediate levels of antigenic relatedness (R approximately 25%) were seen in neutralizing antibodies from cotton rats infected with RSV of the two subgroups. Immunity against the F glycoprotein of subgroup A, induced by vaccinia-A2-F, conferred a high level of protection which was of comparable magnitude against challenge by RSV of either subgroup. In comparison, immunity against the G glycoprotein of subgroup A, induced by vaccinia-A2-G, conferred less complete, but significant, protection. Importantly, in vaccinia-A2-G-immunized animals, suppression of homologous challenge virus replication was significantly greater (13-fold) than that observed for the heterologous virus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHETTI I., HORSFALL F. L., Jr Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J Exp Med. 1950 Nov 1;92(5):441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerlind B., Norrby E. Occurrence of respiratory syncytial virus subtypes A and B strains in Sweden. J Med Virol. 1986 Jul;19(3):241–247. doi: 10.1002/jmv.1890190306. [DOI] [PubMed] [Google Scholar]
- Alling D. W. Tests of relatedness. Biometrika. 1967 Dec;54(3):459–469. [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Tsou C., Hendry R. M., Fernie B. F., Stone Y., McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985 Apr;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- Ball L. A., Young K. K., Anderson K., Collins P. L., Wertz G. W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R., ROIZMAN B., MYERS R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg. 1957 Nov;66(3):281–290. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- COATES H. V., KENDRICK L., CHANOCK R. M. Antigenic differences between two strains of respiratory syncytial virus. Proc Soc Exp Biol Med. 1963 Apr;112:958–964. doi: 10.3181/00379727-112-28221. [DOI] [PubMed] [Google Scholar]
- Coates H. V., Alling D. W., Chanock R. M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966 Mar;83(2):299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Dickens L. E., Buckler-White A., Olmsted R. A., Spriggs M. K., Camargo E., Coelingh K. V. Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4594–4598. doi: 10.1073/pnas.83.13.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Prince G. A., Murphy B. R., Venkatesan S., Chanock R. M., Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez H. B., Hardman N., Keir H. M., Cash P. Antigenic variation between human respiratory syncytial virus isolates. J Gen Virol. 1986 May;67(Pt 5):863–870. doi: 10.1099/0022-1317-67-5-863. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Hendry R. M., Talis A. L., Godfrey E., Anderson L. J., Fernie B. F., McIntosh K. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J Infect Dis. 1986 Feb;153(2):291–297. doi: 10.1093/infdis/153.2.291. [DOI] [PubMed] [Google Scholar]
- Mufson M. A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985 Oct;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Graham B. S., Prince G. A., Walsh E. E., Chanock R. M., Karzon D. T., Wright P. F. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol. 1986 Jun;23(6):1009–1014. doi: 10.1128/jcm.23.6.1009-1014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Elango N., Prince G. A., Murphy B. R., Johnson P. R., Moss B., Chanock R. M., Collins P. L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Koenig D. W., Chanock R. M. Antigenic analysis of a putative new strain of respiratory syncytial virus. J Infect Dis. 1985 Apr;151(4):634–637. doi: 10.1093/infdis/151.4.634. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Horswood R. L., Camargo E., Chanock R. M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978 Dec;93(3):771–791. [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Ball L. A., Young K. K., Furze J., Wertz G. W. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol. 1986 Nov;60(2):607–613. doi: 10.1128/jvi.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Brandriss M. W., Schlesinger J. J. Purification and characterization of the respiratory syncytial virus fusion protein. J Gen Virol. 1985 Mar;66(Pt 3):409–415. doi: 10.1099/0022-1317-66-3-409. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus. J Gen Virol. 1984 Apr;65(Pt 4):761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]


