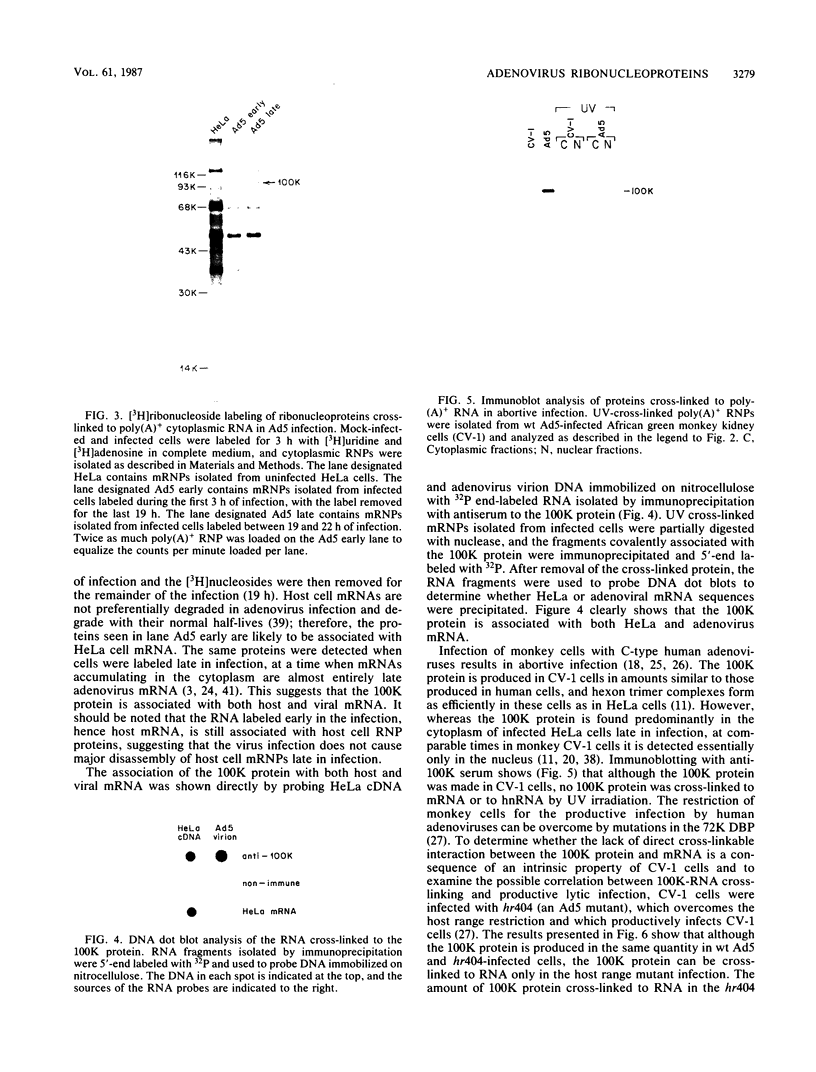
Abstract
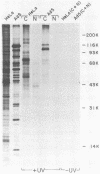
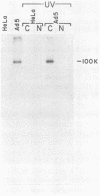
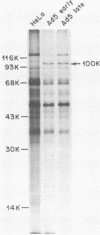
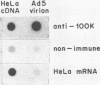
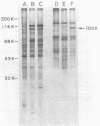
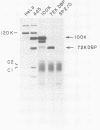
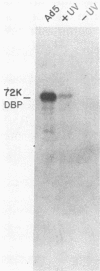
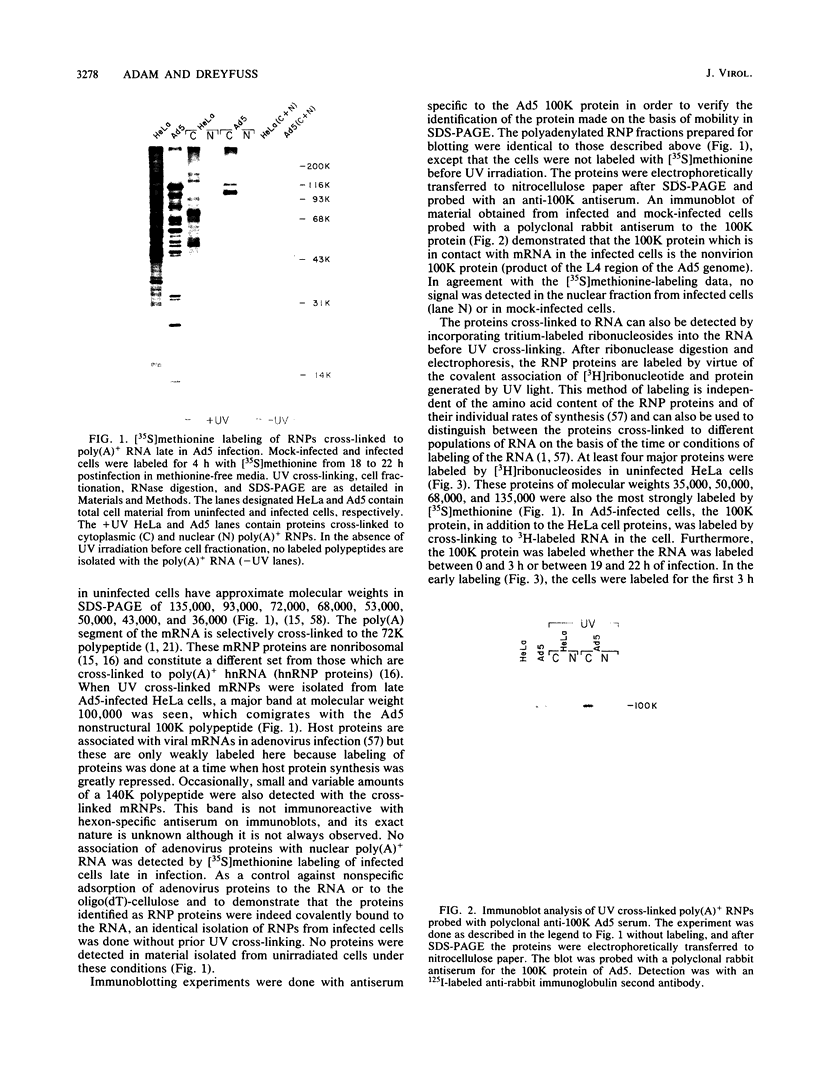
The proteins that interact with cytoplasmic and nuclear polyadenylated RNA in adenovirus type 5 (Ad5) infection of HeLa cells were examined by UV-induced RNA-protein cross-linking in intact cells. The Ad5 100-kilodalton late nonvirion protein (100K protein) was cross-linked to both host and viral polyadenylated cytoplasmic RNA (mRNA). The cross-linking of the 100K protein to mRNA appears to correlate with productive infection, because the protein is not cross-linked to mRNA in abortive infection of wild-type Ad5 in monkey cells (CV-1) even though normal amounts of it are produced. However, when CV-1 cells are infected with Ad5 hr404, and Ad5 mutant which overcomes the host restriction to wild-type Ad5 infection in these cells, the 100K protein is cross-linked to mRNA. To identify and obtain antibodies to RNA-contacting proteins, a mouse was immunized with oligo(dT)-selected cross-linked RNA-protein complexes from Ad5-infected cells and the serum was used for immunoblotting experiments. It was found that in addition to the 100K protein, the Ad5 72K DNA-binding protein is also associated with RNA in the infected cells. The 72K DNA-binding protein is cross-linked to polyadenylated nuclear RNA sequences. These findings indicate that adenovirus proteins interact with RNAs in the infected cell and suggest possible mechanisms for the effects of the virus on mRNA metabolism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Choi Y. D., Dreyfuss G. Interaction of mRNA with proteins in vesicular stomatitis virus-infected cells. J Virol. 1986 Feb;57(2):614–622. doi: 10.1128/jvi.57.2.614-622.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich A., Nevins J. R. The stability of early adenovirus mRNA is controlled by the viral 72 kd DNA-binding protein. Cell. 1981 Nov;26(3 Pt 1):371–379. doi: 10.1016/0092-8674(81)90206-3. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Darnell J. E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985 Oct;5(10):2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Russell W. C. Adenovirus polypeptide synthesis in the presence of non-replicating poliovirus. J Gen Virol. 1974 Aug;24(2):261–279. doi: 10.1099/0022-1317-24-2-261. [DOI] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J Virol. 1967 Oct;1(5):843–850. doi: 10.1128/jvi.1.5.843-850.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Sharp P. A. Aberrant distribution of human adenovirus type 2 late proteins in monkey kidney cells. J Virol. 1983 Apr;46(1):302–306. doi: 10.1128/jvi.46.1.302-306.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Sharp P. A. Analysis of Ad5 hexon and 100K ts mutants using conformation-specific monoclonal antibodies. Virology. 1983 Aug;129(1):137–154. doi: 10.1016/0042-6822(83)90402-6. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Sharp P. A. Assembly of adenovirus major capsid protein is mediated by a nonvirion protein. Cell. 1982 Dec;31(2 Pt 1):407–415. doi: 10.1016/0092-8674(82)90134-9. [DOI] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Monoclonal antibody characterization of the C proteins of heterogeneous nuclear ribonucleoprotein complexes in vertebrate cells. J Cell Biol. 1984 Dec;99(6):1997–1204. doi: 10.1083/jcb.99.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleghon V. G., Klessig D. F. Association of the adenovirus DNA-binding protein with RNA both in vitro and in vivo. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8947–8951. doi: 10.1073/pnas.83.23.8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Choi Y. D., Adam S. A. Characterization of heterogeneous nuclear RNA-protein complexes in vivo with monoclonal antibodies. Mol Cell Biol. 1984 Jun;4(6):1104–1114. doi: 10.1128/mcb.4.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G. Structure and function of nuclear and cytoplasmic ribonucleoprotein particles. Annu Rev Cell Biol. 1986;2:459–498. doi: 10.1146/annurev.cb.02.110186.002331. [DOI] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. Post-transcriptional restriction of human adenovirus expression in monkey cells. J Virol. 1975 May;15(5):1256–1261. doi: 10.1128/jvi.15.5.1256-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Beltz G. A., Linzer D. I. Synthesis and processing of simian virus 40-specific RNA in adenovirus-infected, simian virus 40-transformed human cells. J Mol Biol. 1983 Jun 25;167(2):335–359. doi: 10.1016/s0022-2836(83)80339-8. [DOI] [PubMed] [Google Scholar]
- Gambke C., Deppert W. Specific complex of the late nonstructural 100,000-dalton protein with newly synthesized hexon in adenovirus type 2-infected cells. Virology. 1983 Jan 15;124(1):1–12. doi: 10.1016/0042-6822(83)90285-4. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R., Carroll E., 3rd Reconstitution of functional mRNA-protein complexes in a rabbit reticulocyte cell-free translation system. Mol Cell Biol. 1985 Feb;5(2):342–351. doi: 10.1128/mcb.5.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S. I., Green M. Multiple methylated cap sequences in adenovirus type 2 early mRNA. J Virol. 1976 Nov;20(2):425–435. doi: 10.1128/jvi.20.2.425-435.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman R. S., Ginsberg H. S. Characterization of a temperature-sensitive, hexon transport mutant of type 5 adenovirus. J Virol. 1976 Aug;19(2):643–658. doi: 10.1128/jvi.19.2.643-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili K., Weinmann R. Shut-off of actin biosynthesis in adenovirus serotype-2-infected cells. J Mol Biol. 1984 Jun 5;175(4):453–468. doi: 10.1016/0022-2836(84)90179-7. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Anderson C. W. Block to multiplication of adenovirus serotype 2 in monkey cells. J Virol. 1975 Dec;16(6):1650–1668. doi: 10.1128/jvi.16.6.1650-1668.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F., Chow L. T. Incomplete splicing and deficient accumulation of the fiber messenger RNA in monkey cells infected by human adenovirus type 2. J Mol Biol. 1980 May 15;139(2):221–242. doi: 10.1016/0022-2836(80)90306-x. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leibowitz J., Horwitz M. S. Synthesis and assembly of adenovirus polypeptides. III. Reversible inhibition of hexon assembly in adenovirus type 5 temperature-sensitive mutants. Virology. 1975 Jul;66(1):10–24. doi: 10.1016/0042-6822(75)90175-0. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974 Jun 25;86(2):451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Pederson T. Nuclear ribonucleoprotein particles probed in living cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2208–2212. doi: 10.1073/pnas.78.4.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Setyono B., Greenberg J. R., Pederson T. Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol. 1981 Aug;90(2):380–384. doi: 10.1083/jcb.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Koczot F. Sequence of methylated nucleotides at the 5'-terminus of adenovirus-specific RNA. J Virol. 1976 Feb;17(2):385–392. doi: 10.1128/jvi.17.2.385-392.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Oosterom-Dragon E. A., Ginsberg H. S. Purification and preliminary immunological characterization of the type 5 adenovirus, nonstructural 100,000-dalton protein. J Virol. 1980 Mar;33(3):1203–1207. doi: 10.1128/jvi.33.3.1203-1207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S., Moore M., Logan J., Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986 Feb;6(2):470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Sarnow P., Duprey E., Levine A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983 Jul 30;128(2):480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Ennis H. L., Cohen P. S. Translational control of vesicular stomatitis virus protein synthesis: isolation of an mRNA-sequestering particle. J Virol. 1982 Dec;44(3):932–938. doi: 10.1128/jvi.44.3.932-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Siekierka J., Ennis H. L., Cohen P. S. Inhibition of protein synthesis in vesicular stomatitis virus infected Chinese hamster ovary cells: role of virus mRNA-ribonucleoprotein particle. Biochemistry. 1984 May 22;23(11):2407–2411. doi: 10.1021/bi00306a014. [DOI] [PubMed] [Google Scholar]
- Russel W. C., Newman C., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5--serology. J Gen Virol. 1972 Dec;17(3):265–279. doi: 10.1099/0022-1317-17-3-265. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S., Salditt-Georgieff M., Bachenheimer S., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. J. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976 Mar;3(3):749–765. doi: 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Ginsberg H. S. Antibody to the type 5 adenovirus hexon polypeptide: detection of nascent polypeptides in the cytoplasm of infected KB cells. Intervirology. 1974;4(4):226–236. doi: 10.1159/000149967. [DOI] [PubMed] [Google Scholar]
- Sundquist B., Persson T., Lindberg U. Characterization of mRNA-protein complexes from mammalian cells. Nucleic Acids Res. 1977 Apr;4(4):899–915. doi: 10.1093/nar/4.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasseron-De Jong J. G., Brouwer J., Rietveld K., Zoetemelk C. E., Bosch L. Messenger ribonucleoprotein complexes in human KB cells infected with adenovirus type 5 contain tightly bound viral-coded '100K' proteins. Eur J Biochem. 1979 Oct;100(1):271–283. doi: 10.1111/j.1432-1033.1979.tb02058.x. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Ustacelebi S., Williams J. F. Temperature-sensitive mutants of adenovirus defective in interferon induction at non-permissive temperature. Nature. 1972 Jan 7;235(5332):52–53. doi: 10.1038/235052a0. [DOI] [PubMed] [Google Scholar]
- Van Eekelen C. A., Mariman E. C., Reinders R. J., Van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with host cell proteins. Eur J Biochem. 1981 Oct;119(3):461–467. doi: 10.1111/j.1432-1033.1981.tb05630.x. [DOI] [PubMed] [Google Scholar]
- Van der Marel P., Tasseron-de Jong J. G., Bosch L. The proteins associated with mRNA from uninfected and adenovirus type 5-infected KB cells. FEBS Lett. 1975 Mar 1;51(1):330–334. doi: 10.1016/0014-5793(75)80919-7. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Riemen T., van Eekelen C. A. Host proteins are associated with adenovirus specific mRNA in the cytoplasm. FEBS Lett. 1982 Aug 16;145(1):62–71. doi: 10.1016/0014-5793(82)81207-6. [DOI] [PubMed] [Google Scholar]