Abstract
Progesterone-induced meiotic maturation of Xenopus oocytes requires the synthesis of new proteins, such as Mos and cyclin B. Synthesis of Mos is thought to be necessary and sufficient for meiotic maturation; however, it has recently been proposed that newly synthesized proteins binding to p34cdc2 could be involved in a signaling pathway that triggers the activation of maturation-promoting factor. We focused our attention on cyclin B proteins because they are synthesized in response to progesterone, they bind to p34cdc2, and their microinjection into resting oocytes induces meiotic maturation. We investigated cyclin B accumulation in response to progesterone in the absence of maturation-promoting factor–induced feedback. We report here that the cdk inhibitor p21cip1, when microinjected into immature Xenopus oocytes, blocks germinal vesicle breakdown induced by progesterone, by maturation-promoting factor transfer, or by injection of okadaic acid. After microinjection of p21cip1, progesterone fails to induce the activation of MAPK or p34cdc2, and Mos does not accumulate. In contrast, the level of cyclin B1 increases normally in a manner dependent on down-regulation of cAMP-dependent protein kinase but independent of cap-ribose methylation of mRNA.
INTRODUCTION
Xenopus fully grown oocytes are naturally arrested in prophase of the first meiotic division (prophase I). In response to progesterone, they undergo meiotic maturation and then arrest a second time in metaphase of the second meiotic division (metaphase II) until fertilization. The steroid hormone progesterone induces with a lag of 3–5 h activation of maturation-promoting factor (MPF), breakdown of the germinal vesicle (GVBD), and entry into M-phase of the first meiotic division (see Masui and Clarke, 1979, for review). MPF is a protein kinase composed of a catalytic subunit, p34cdc2 (Dunphy et al., 1988; Gautier et al., 1988; Draetta et al., 1989; Meijer et al., 1989), and a regulatory subunit, cyclin B (Gautier et al., 1990), that accumulates during terminal oocyte growth to form a complex called preMPF (Wasserman and Masui, 1975; Gerhart et al., 1984; Gautier and Maller, 1991). PreMPF is kept inactive by inhibitory phosphorylations on threonine 14 and tyrosine 15 of the p34cdc2 subunit. The conversion of preMPF into active MPF occurs several hours after progesterone stimulation, just before GVBD; it depends on activation of the protein phosphatase Cdc25, which catalyzes the dephosphorylation of threonine 14 and tyrosine 15 of p34cdc2. At about the same time, i.e., around GVBD, MAPK becomes fully activated (Gotoh et al., 1991; Matsuda et al., 1992; Roy et al., 1996). Little is known about the transduction pathway that connects the initial effects of progesterone to activation of preMPF.
It is well established that progesterone-induced maturation depends on the synthesis of new proteins and is inhibited by the cAMP pathway (Maller and Krebs, 1977). The protein kinase Mos is the only newly synthesized protein that has been shown to be necessary for progesterone-induced maturation (Sagata et al., 1988). Mos is a MAP kinase kinase kinase that leads to the activation of MAPK through the activation of MAP kinase kinase (also called MEK) (Nebreda et al., 1993; Posada et al., 1993; Shibuya and Ruderman, 1993); therefore, it is generally proposed that in ovo the translation of stored mos mRNA induces MAPK activation in maturing oocytes. In the absence of p34cdc2 activity, Mos is synthesized but does not attain normal levels (Nebreda et al., 1995). This raises the question of whether the accumulation of Mos is stimulated by p34cdc2 activity. In theory, the accumulation of a protein absolutely required for preMPF activation must depend on progesterone stimulation and be independent of p34cdc2 activity. It is important, therefore, to distinguish the newly made proteins that accumulate during the lag period before p34cdc2 activation from those whose accumulation is stimulated downstream of p34cdc2 activation.
We report here a direct approach for identifying proteins that accumulate independently of p34cdc2 activity after progesterone stimulation. For this purpose, we microinjected into prophase oocytes recombinant p21cip1, a well-known inhibitor of cdk/cyclin complexes (Xiong et al., 1993). We show that microinjected p21cip1 binds endogenous p34cdc2/cyclin complexes and prevents their activation in ovo. Therefore, we used p21cip1 as a tool to study the accumulation of proteins such as cyclin B1, cyclin B2, and Mos under conditions in which progesterone-induced MPF activation and GVBD were abolished.
MATERIALS AND METHODS
Materials
Xenopus laevis adult females (Centre National de la Recherche Scientifique, Rennes, France) were bred and maintained under laboratory conditions. Reagents, unless otherwise specified, were from Sigma (Saint Quentin Fallavier, France).
Purification of Recombinant Proteins
Cip1 cloned into the NcoI site of pGEX-KG (a kind gift of Dr. Tim Hunt) was transfected into TG1 cells. Expression of the GST-p21cip1 fusion protein was induced by 0.1 mM isopropyl-β-d-thiogalactopyranoside for 3 h at room temperature. After centrifugation at 10,000 × g for 15 min at 4°C, the pellets were frozen. Bacteria were lysed in 50 mM Tris-HCl, pH 7.3, 0.1 M NaCl, 1 mM EDTA, 1 mM EGTA, 5 mM benzamidine, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (Pentapharm, Basel, Switzerland), 1 mg/ml lysozyme (Boehringer Mannheim, Indianapolis, IN) by sonication in the presence of detergents (0.5% Triton, 0.5% NP40). After ultracentrifugation (100,000 × g, 4°C, 1 h), the supernatant containing recombinant p21cip1 was loaded onto an equilibrated glutathione-agarose column, washed, and eluted with 20 mM Tris-HCl, pH 9, 0.5 M NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 10 mM glutathione. The purified proteins were concentrated with a Microcon-50 (Millipore, Saint Quentin en Yvelines, France) to a final concentration of 0.75 mg/ml. MBP-Mos was purified as described by Roy et al. (1996).
Synthesis of [35S]Methionine-labeled Cyclin B1
Twenty micrograms of circular DNA containing the sequence coding for Xenopus cyclin B1 were incubated for 3 h at 30°C in 200 μl of TNT-coupled reticulocyte lysate system containing T7 polymerase (Promega, Charbonnieres, France) in the presence of 400 μCi of [35S]methionine (New England Nuclear, Boston, MA). The newly synthesized protein was concentrated and washed on Microcon-10 (Millipore) before injection into oocytes.
Oocyte Treatment
Isolated oocytes were prepared and maintained as described (Jessus et al., 1987). Fully grown oocytes, referred to as “prophase oocytes,” were injected or not with p21cip1 to an internal concentration of 1 μM. One hour after microinjection, oocytes were induced to mature either by the addition of 1 μM progesterone in the medium, by MPF transfer, by microinjection of 50 nl of 10−5 M okadaic acid (OA) (ICN, Orsay, France), by microinjection of the recombinant thermostable inhibitor of the catalytic subunit of cAMP-dependent protein kinase (PKI) from rabbit muscle (8 pmol/oocyte), or by microinjection of recombinant MBP-Mos (50 μg/oocyte).
In other experiments, maturation was inhibited by microinjection of 10 ng of the catalytic subunit of PKA (PKAc) per oocyte (Promega) 1 h before the addition of progesterone, by preincubation in the presence of 0.75 mM S-isobutylthioadenosine (SIBA) for 2 h, or by preincubation in the presence of 100 μg/ml cycloheximide for 1 h.
Maturation was monitored by the appearance of a white spot at the animal pole of the oocyte. Oocytes were collected when 100% GVBD was reached in control oocytes, or at metaphase II (2 h after 100% GVBD in progesterone controls), or at the indicated times. Oocytes matured in vitro (metaphase II) were injected or not with p21cip1 to an internal concentration of 1 μM in the presence of 100 mM EGTA. Oocytes were homogenized at 4°C in 5 volumes of extraction buffer (80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 1 mM DTT, pH 7.3, 25 μg/ml leupeptin and aprotinin, 10 μg/ml pepstatin, 1 mM benzamidine, 1 μM 4-(2 aminoethyl)-benzenesulfonyl fluoride [Pentapharm]) and centrifuged at 7000 rpm for 15 min at 4°C. Clear supernatant was used for Western blot analysis or for recovery of proteins on glutathione-agarose beads.
Histone H1 Kinase Assays
The supernatant (15 μl, i.e., three oocytes) was collected for p13suc1 binding followed by histone H1 kinase assays in the presence of [γ-32P]ATP (New England Nuclear) according to Jessus et al. (1991).
Immunoblotting
Western blotting was performed with anti-Mos, anti-MAPK (Santa Cruz Biotech, Santa Cruz, CA), anti-p34cdc2 (a kind gift from Dr. Tim Hunt, Imperial Research Fond, London, United Kingdom), anti-cyclin B1, anti-cyclin B2, or anti-cyclin A antibodies. The antisera raised in sheep against Xenopus cyclins A, B1, and B2 have been described by Gautier et al. (1990); antisera to cyclins B1 and B2 were blot purified using recombinant cyclin B1 and B2 as described (Olmsted, 1981; Rempel et al., 1995). Proteins were subjected to electrophoresis in Laemmli buffer (Laemmli, 1970) on a 12.5% SDS-PAGE Anderson gel (Anderson et al., 1973) or on a 15% SDS-PAGE Laemmli gel (Laemmli, 1970) and then transferred to nitrocellulose filters (Schleicher & Schull, Ecquevilly, France). The proteins of interest were visualized by use of the appropriate primary antibody, HRP-conjugated secondary antibody (Jackson Immunoresearch, West Grove, PA), and renaissance chemoluminescence reagent (New England Nuclear). We observed that the amount of cyclin B1 detected in prophase-arrested oocytes varied from female to female. Cyclin B1 was resolved as two bands on 12.5% Anderson gels and as one band on 15% Laemmli gels (see Figure 6, A and B).
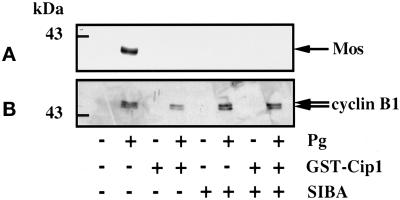
Figure 6.
Effect of SIBA on the accumulation of cyclin B1. Thirty prophase oocytes, microinjected or not with p21cip1, were cultured with or without of 0.75 mM SIBA for 2 h. Then, progesterone (Pg) was added or not and oocytes were cultured overnight. Oocytes were collected, homogenized, and analyzed by immunoblotting for Mos (A) or cyclin B1 (B). An amount equivalent to three oocytes was loaded on each lane.
Binding of GST-p21cip1 to Glutathione-Agarose Beads
Fifteen milligrams of glutathione-agarose beads saturated with extraction buffer containing 10% BSA was added to a supernatant prepared from 30 oocytes. After incubation for 2 h, the beads were washed three times with 20 mM Tris, 5 mM EDTA, 1% Triton X-100 containing 100 mM NaCl, then 1 M NaCl, and finally 100 mM NaCl. Proteins bound to the beads were eluted with SDS-sample buffer, subjected to electrophoresis, and analyzed by Western blotting.
Autoradiography of [35S]Methionine-labeled Cyclin B1
Oocytes injected with [35S]methionine-labeled cyclin B1 were collected at different times, homogenized, and analyzed by autoradiography on a 12.5% SDS-PAGE Anderson gel (Anderson et al., 1973). An amount equivalent to four oocytes was loaded on each lane.
RESULTS
p21cip1 Inhibits Progesterone-induced Maturation
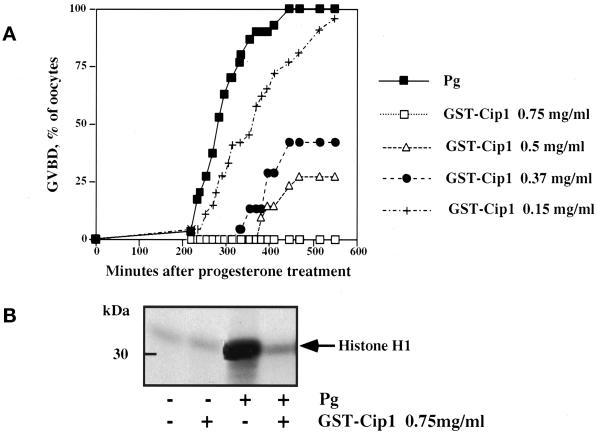
Human p21cip1 is an inhibitor of all cdk/cyclin complexes (Xiong et al., 1993). To determine whether p21cip1 could inhibit oocyte maturation, recombinant p21cip1 prepared as a GST fusion protein (GST-p21cip1) was microinjected into fully grown prophase oocytes. At a final intracellular concentration of 1 μM (0.75 mg/ml in the pipette), p21cip1 totally inhibited progesterone-induced GVBD (Figure 1A). This effect was obtained with oocytes isolated from more than 30 different females and was dose dependent, with a 50% inhibitory concentration of ∼0.4 μM. The activity of p34cdc2 was assayed by measuring histone H1 kinase activity in progesterone-treated control and p21cip1-injected oocytes (Figure 1B). Histone H1 kinase activity was high 2 h after GVBD in control oocytes. In contrast, in p21cip1-injected oocytes, preMPF activation did not occur and activity remained at the basal level, comparable to that in prophase control oocytes. Histone H1 kinase activation was blocked in p21cip1-injected oocytes for as long as 24 h in the continuous presence of progesterone.
Figure 1.
Effect of p21cip1 microinjection on meiotic maturation and preMPF activation in Xenopus oocytes. Thirty prophase oocytes were microinjected or not with 50 nl of p21cip1 at various concentrations (50 nl of the 0.75 mg/ml solution results in an internal concentration of p21cip1 of ∼1 μM). One hour after microinjection, oocytes were treated with 10−6 M progesterone (Pg), cultured, and scored for GVBD (A). Histone H1 kinase activity was measured 2 h after GVBD in control and progesterone-treated oocytes injected or not with GST-Cip1, as indicated (B).
p21cip1 Interacts with p34cdc2/Cyclin Complexes and Inhibits MPF Autoamplification but Not the Mos-induced MAPK Pathway
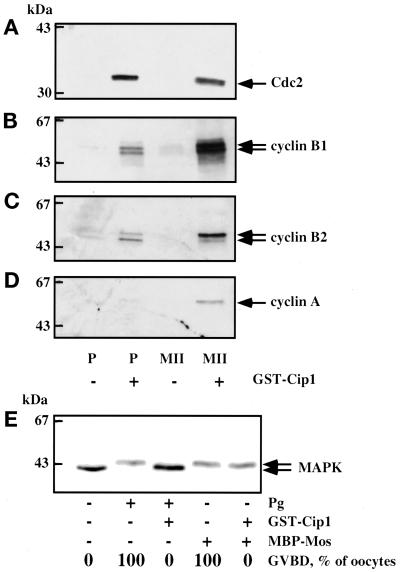
We next examined whether injected p21cip1 inhibits p34cdc2 activation and GVBD by directly binding to endogenous p34cdc2/cyclin complexes. p21cip1 was microinjected into prophase oocytes; 1 h later, oocytes were collected, homogenized, and centrifuged, and the supernatant was incubated in the presence of glutathione-agarose beads. Proteins bound to the beads were then analyzed by immunoblotting with various antibodies. Microinjected p21cip1 was recovered in association with the glutathione beads. p34cdc2, cyclin B1, and cyclin B2 were also bound to the beads (Figure 2, A–C). A similar experiment was also performed with oocytes matured in vitro and arrested in metaphase II. As in prophase oocytes, p34cdc2/cyclin B2 and p34cdc2/cyclin B1 were recovered on the beads (Figure 2, A–C); as expected, cyclin A, which is absent in prophase oocytes and is synthesized de novo during maturation, was also bound to the beads (Figure 2D). These results indicate that p21cip1 acts in ovo by direct binding to active or inactive p34cdc2/cyclin complexes.
Figure 2.
p21cip1 interacts with p34cdc2/cyclin complexes and does not inhibit the MAPK pathway. (A–D) Recovery of complexes bound to p21cip1. Thirty prophase (P) or metaphase II (MII) oocytes were microinjected or not with p21cip1 to an internal concentration of 1 μM and cultured for 1 h. After homogenization of oocytes, glutathione-agarose beads were added to the clear supernatant. Beads were washed, and bound proteins were electrophoresed and subjected to Western blot analysis for p34cdc2 (A), cyclin B1 (B), cyclin B2 (C), and cyclin A (D). (E) Effect of p21cip1 on the MAPK electrophoretic mobility shift induced by injection of MBP-Mos. Twenty prophase oocytes were microinjected or not with p21cip1 (1 μM). One hour later, they were microinjected with 50 μg of recombinant MBP-Mos or treated with progesterone (Pg). Oocytes were collected at the time of 100% GVBD in MBP-Mos–injected oocytes. An amount equivalent to three oocytes was subjected to Western blot analysis for MAPK.
We next verified whether p21cip1 could inhibit the MAPK pathway. When recombinant MBP-Mos was injected into p21cip1-treated oocytes, activation of MPF and GVBD were blocked. However, the electrophoretic mobility shift of MAPK was still observed (Figure 2E). Moreover, it has been shown that the activity of oocyte MAPK measured by an in-gel assay was not affected by the presence of p21cip1 (Karaiskou et al., 1998). These results show that p21cip1 does not inhibit components of the MAPK pathway.
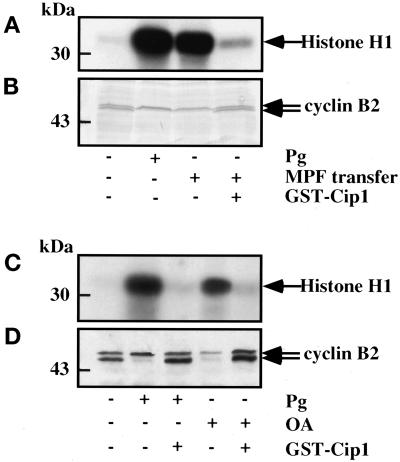
If p21cip1 acts by direct binding to MPF, then it should also inhibit meiotic maturation induced by MPF transfer because it also requires activation of preMPF. When a small amount of cytoplasm taken from matured oocytes is microinjected into prophase oocytes, p34cdc2 activation and GVBD occur less than 3 h later (Masui and Markert, 1971). When oocytes were first microinjected with p21cip1 and then 1 h later with 50 nl of cytoplasm taken from a matured oocyte, GVBD did not occur, H1 kinase activity was not increased, and the electrophoretic mobility shift of cyclin B2 was blocked (Figure 3, A and B).
Figure 3.
Effect of p21cip1 on MPF autoamplification. Twenty prophase oocytes were microinjected or not with p21cip1 (1 μM). One hour later, they were microinjected with either 50 nl of cytoplasm taken from metaphase II oocytes containing active MPF (A and B) or 50 nl of 10−5 M OA (C and D). All control oocytes underwent GVBD, whereas none of the p21cip1-injected oocytes underwent GVBD. Oocytes were collected at the time when 100% GVBD was reached in the control oocytes (3.5 h after MPF transfer or 6 h after OA injection). MPF activity was measured by assaying histone H1 kinase activity (A and C), and an amount equivalent to three oocytes was subjected to Western blot analysis for cyclin B2 (B and D). Pg, progesterone.
OA is an inhibitor of types 1 and 2A Ser/Thr protein phosphatases (Bialojan and Takai, 1988). Microinjection of 50 nl of 10−5 M OA into prophase oocytes induces rapid p34cdc2 activation (Goris et al., 1989) and a subsequent cytologically abnormal GVBD (Rime et al., 1990). It has been proposed that microinjected OA, by promoting phosphorylation/activation of Cdc25, initiates the positive feedback loop that controls activation of p34cdc2 in ovo (Izumi and Maller, 1993). When OA was microinjected into p21cip1-injected oocytes, p34cdc2 activation and the electrophoretic mobility shift of cyclin B2 were totally blocked for at least 8 h (Figure 3, C and D).
Progesterone Induces Accumulation of Cyclin B1 in p21cip1-microinjected Oocytes
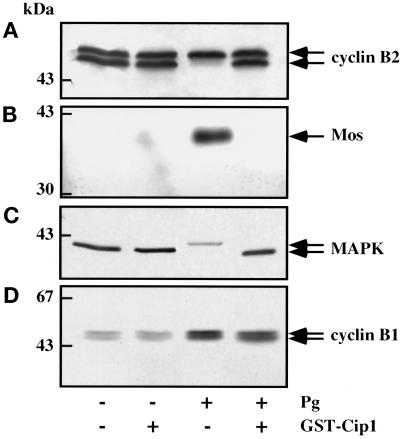
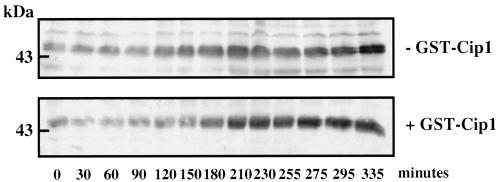
As shown above, microinjection of recombinant p21cip1 inhibited preMPF activation induced by progesterone. The electrophoretic mobility shift of cyclin B2, which normally correlates with p34cdc2 activation (Gautier et al., 1990; Roy et al., 1996), was also totally blocked (Figure 4A). Although the synthesis of Mos can be detected before the conversion of preMPF to active MPF (Sagata et al., 1989; Nebreda et al., 1995), accumulation of Mos does not become detectable before GVBD (Roy et al., 1996). Surprisingly, in p21cip1-injected oocytes, the level of Mos did not increase after progesterone stimulation (Figure 4B); as expected from the absence of Mos under these conditions, the electrophoretic mobility of MAPK was not retarded (Figure 4C). Therefore, the progesterone-dependent accumulation of Mos does not occur in the absence of p34cdc2 activity. Unexpectedly, however, in p21cip1-inhibited oocytes, cyclin B1 accumulated normally after progesterone treatment (Figure 4D). The time course of the accumulation of cyclin B1 in control progesterone-treated oocytes and in p21cip1-injected progesterone-treated oocytes was similar (Figure 5). Interestingly, in both control (Kobayashi et al., 1991) and p21cip1-inhibited oocytes, the accumulation of cyclin B1 after progesterone stimulation takes place after a lag period of several hours and is thus not an early effect of the hormone. These results show that accumulation of cyclin B1 is regulated differently from that of Mos in response to progesterone. In particular, the amount of cyclin B1 is up-regulated by progesterone independently of p34cdc2 and MAPK activities.
Figure 4.
Effect of p21cip1 microinjection on the electrophoretic mobility of cyclin B2 (A), accumulation of Mos (B), electrophoretic mobility of MAPK (C), and accumulation of cyclin B1 (D). Twenty prophase oocytes were microinjected or not with p21cip1 to an internal concentration of 1 μM, and progesterone (Pg) was added 1 h later. Oocytes were collected 2 h after 100% GVBD was reached in progesterone control oocytes and homogenized for Western blot analysis with the indicated antibodies. An amount equivalent to three oocytes was loaded on each lane.
Figure 5.
Time course of the accumulation of cyclin B1. Two hundred prophase oocytes were microinjected (bottom) or not (top) with p21cip1 to an internal concentration of 1 μM. One hour later, they were incubated in the presence of progesterone. Groups of 10 oocytes were collected at the indicated times after progesterone addition and homogenized for Western blot analysis with the anti-cyclin B1 antibody. An amount equivalent to three oocytes was loaded on each lane.
It has been reported that SIBA, a methyltransferase inhibitor, prevents mRNA cap-ribose methylation, Mos synthesis, and oocyte maturation (Kuge et al., 1998). Therefore, we examined the effect of SIBA on the accumulation of cyclin B1. Oocytes were preincubated or not in the presence of 750 μM SIBA for 2 h, p21cip1 was then microinjected, and 1 h later progesterone was added or not in the continuous presence of SIBA. As expected, meiotic maturation induced by progesterone was totally prevented by SIBA. As reported by Kuge et al. (1998), accumulation of Mos was blocked (Figure 6A); however, accumulation of cyclin B1 was still observed (Figure 6B). This result shows that the increased level of cyclin B1 does not depend on new cap-ribose methylation of mRNAs, not even that of mos mRNA.
Progesterone Does Not Change the Stability of Microinjected Cyclin B1
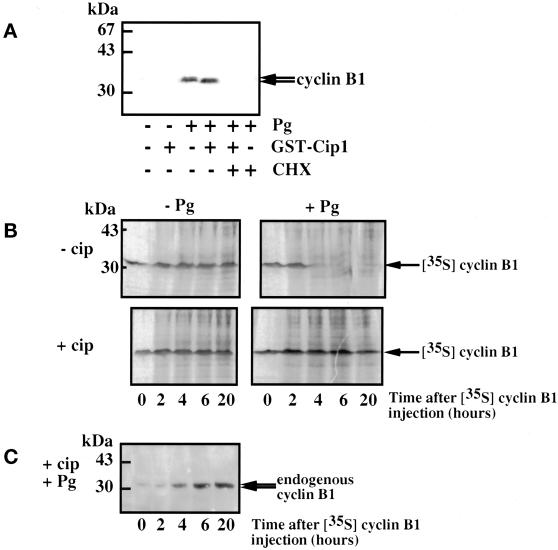
We then addressed the question of whether cyclin B1 accumulation was the consequence of either the increased rate of synthesis and/or a decrease in the rate of degradation. Oocytes were first incubated in the presence of cycloheximide, a strong inhibitor of protein synthesis. They were then injected or not with p21cip1, and 1 h later they were incubated in the presence of progesterone. As expected, cyclin B1 did not accumulate (Figure 7A). This finding shows that cyclin B1 accumulation is a consequence of a new synthesis and does not result from decreased degradation.
Figure 7.
Synthesis and degradation of cyclin B1. (A) Twenty prophase oocytes were microinjected or not with p21cip1 (1 μM) and incubated or not in the presence of cycloheximide (CHX) at 100 μg/ml for 1 h before progesterone (Pg) addition. Oocytes were cultured overnight, collected, homogenized, and analyzed by Western blotting for cyclin B1. An amount equivalent to two oocytes was loaded on each lane. (B) Stability of microinjected cyclin B1. Seventy oocytes were microinjected or not with p21cip1 (1 μM), microinjected with [35S]methionine-labeled cyclin B1, and incubated in the presence or absence of progesterone. Ten oocytes of each group were collected at different times, homogenized, and analyzed by autoradiography. An amount equivalent to four oocytes was loaded on each lane. (C) Extracts from B (lower left panel) were analyzed by Western blotting for cyclin B1. An amount equivalent to two oocytes was loaded on each lane.
To estimate the in ovo stability of cyclin B1, [35S]methionine-labeled cyclin B1 was injected into prophase oocytes in the presence or in the absence of p21cip1, stimulated or not with progesterone. We injected trace amounts of radioactive cyclin B1 that were not able to induce meiotic maturation. In prophase oocytes, ectopic cyclin B1 remained stable from more than 20 h (Figure 7B, upper left panel). When oocytes were first microinjected with radioactive cyclin B1 and then treated with progesterone, cyclin B1 was stable until GVBD, after which it was abruptly degraded (Figure 7B, upper right panel). This result demonstrates that the ectopic protein is the target of the anaphase-promoting complex (APC), which is activated at the metaphase/anaphase transition in meiosis I and promotes the degradation of cyclins (Furuno et al., 1994; Roy et al., 1996; Thibier et al., 1997). Radioactive microinjected cyclin B1 was as stable in p21cip1-injected oocytes whether progesterone was added or not (Figure 7B, lower panels). Although exogenous radioactive cyclin B1 levels remained stable in oocytes treated with progesterone in the presence of p21cip1, it was ascertained by Western blotting that under these conditions endogenous cyclin B1 accumulated after progesterone stimulation (Figure 7C). These results suggest that cyclin B1 accumulation induced by progesterone does not involve a change in the stability of cyclin B1.
PKA Inhibits the Accumulation of Cyclin B1
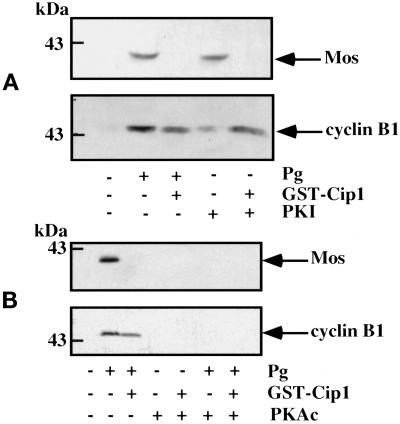
The decrease in cAMP concentration and the subsequent inactivation of PKA are the first early events known to be induced by progesterone. To evaluate whether cyclin B1 accumulation is regulated by PKA activity, we microinjected the thermostable inhibitor of the catalytic subunit of PKA (PKI) into oocytes. Under these conditions, PKI induces 100% of the oocytes to undergo maturation (Maller and Krebs, 1977; Huchon et al., 1981). However, coinjection of p21cip1 blocked the ability of PKI to induce maturation. It also blocked the accumulation of Mos induced by PKI, but the increase in cyclin B1 was not affected (Figure 8A). The inhibition of PKA by progesterone treatment, therefore, is sufficient to promote the accumulation of cyclin B1. To determine whether this inhibition is also necessary, 10 ng of PKAc was microinjected per oocyte. Under these conditions, PKAc inhibited progesterone-induced maturation, and the accumulation of both Mos and cyclin B1 was prevented (Figure 8B). These results clearly show that the accumulation of cyclin B1 requires a decrease in the activity of PKA.
Figure 8.
Accumulation of cyclin B1 is down-regulated by PKA. (A) PKI microinjection. Thirty prophase oocytes were microinjected or not with p21cip1 to an internal concentration of 1 μM. One hour later, they were microinjected with 8 pmol of PKI per oocyte. Control oocytes were treated with progesterone (Pg). Oocytes were cultured overnight, collected, homogenized, and analyzed by Western blotting for Mos (top) or cyclin B1 (bottom). An amount equivalent to two oocytes was loaded on each lane. (In this experiment, cyclin B1 was resolved as a single band on a 15% Laemmli gel.) (B) PKAc microinjection. Thirty prophase oocytes were microinjected or not with p21cip1 to an internal concentration of 1 μM and/or with 10 ng of PKAc per oocyte. One hour later, progesterone was added or not and oocytes were cultured for 5.5 h (50% GVBD occurred at 4 h). Oocytes were collected, homogenized, and analyzed by Western blotting for Mos (top) or cyclin B1 (bottom). An amount equivalent to two oocytes was loaded on each lane. (In this experiment, cyclin B1 was resolved as a single band on a 15% Laemmli gel.)
MPF Transfer or OA Induces Accumulation of Cyclin B1
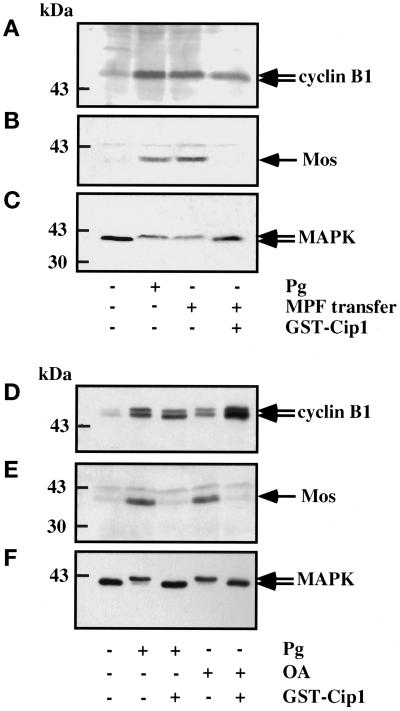
Because MPF transfer is known to induce all the events of meiotic maturation (Masui and Markert, 1971), it was interesting to investigate the effects of MPF transfer on cyclin B1 accumulation. As shown in Figure 9A, when cytoplasm taken from matured oocytes was microinjected into prophase oocytes, cyclin B1 accumulated in the presence or in the absence of p21cip1. In contrast, in the presence of p21cip1 Mos did not accumulate and MAPK remained in its inactive, unshifted form (Figure 9, B and C). Similar results were obtained when OA was microinjected into p21cip1-treated oocytes (Figure 9, D–F). This argues that a phosphorylation step might be involved in the pathway leading to the accumulation of cyclin B1.
Figure 9.
Cyclin B1 and Mos accumulation after MPF transfer or OA microinjection in the presence of p21cip1. Twenty prophase oocytes were microinjected or not with p21cip1 (1 μM). One hour later, they were microinjected with either 50 nl of cytoplasm taken from metaphase II oocytes (A–C) or 50 nl of 10−5 M OA (D–F). Oocytes were collected at the time when 100% GVBD was reached in the control oocytes. An amount equivalent to three oocytes was subjected to Western blot analysis for cyclin B1 (A and D), Mos (B and E), and MAPK (C and F). Pg, progesterone.
DISCUSSION
In this study, we developed a new experimental approach to the study of proteins whose abundance is regulated by progesterone during the lag period preceding p34cdc2 activation in Xenopus oocytes. This approach used the cdk inhibitor p21cip1 to inhibit the activation of preMPF and facilitated the study of the early effects of progesterone. The results show that the level of cyclin B1 is directly regulated by initial progesterone stimulation and does not depend on increased p34cdc2 activity. In contrast, the progesterone-dependent increase of Mos protein was totally inhibited in p21cip1-treated oocytes, indicating that in vivo the activity of p34cdc2 is absolutely required to promote Mos accumulation. Together, these results indicate that regulation of the levels of Mos and cyclin B1 is mediated by two different biochemical pathways.
p21cip1 binds to and inhibits in vitro and in vivo a number of cdk/cyclin complexes (Gu et al., 1993; Xiong et al., 1993; Su et al., 1995). Recently, it was reported that microinjection of p21cip1 into Xenopus oocytes at a final intracellular concentration of 0.1 μM does not inhibit progesterone-induced maturation but totally inhibits the activation of cdk2 that normally occurs after GVBD (Furuno et al., 1997). Because in vitro the affinity of p21cip1 for cdk2/cyclin complexes is higher than its affinity for p34cdc2/cyclin complexes (Su et al., 1995), we microinjected a higher concentration of p21cip1 (1 μM) to inhibit in ovo the activation of p34cdc2/cyclin B complexes. We found that at this concentration p21cip1 binds to endogenous p34cdc2/cyclin B complexes and totally inhibits GVBD and activation of histone H1 kinase. Therefore, under our conditions, p21cip1 acts by binding and inhibiting p34cdc2. This conclusion is strengthened by the observation that p21cip1 also inhibits GVBD after MPF transfer or OA microinjection, i.e., experimental conditions that bypass the early effects of progesterone.
It has been shown that the activity of MAPK depends on a critical threshold of Mos protein (Chen and Cooper, 1997; Ferrell and Bhatt, 1997). The accumulation of Mos leading to this threshold level could result from regulation of the synthesis and/or turnover of the protein. Nebreda et al. (1995) have shown that overexpression of a kinase-inactive p34cdc2 (K33R) or microinjection of the A17 anti-p34cdc2 antibody inhibits progesterone-induced GVBD, activation of p34cdc2, accumulation of Mos, and the MAPK cascade. Interestingly, in the presence of the A17 anti-p34cdc2 antibody, progesterone stimulates the synthesis of 35S-labeled Mos, as detected by immunoprecipitation, but its accumulation is not detectable by immunoblotting (Nebreda et al., 1995). A main finding presented here is that Mos accumulation also does not occur when activation of p34cdc2 is blocked by p21cip1, arguing that the Mos/MAP kinase kinase/MAPK pathway is under the control of p34cdc2 activity (Figure 10). Although not studied directly in this paper, the accumulation of Mos may depend on a change in stability. Indeed, Nishizawa et al. (1993) reported that phosphorylation at a proline-directed site near the N terminus might regulate stability.
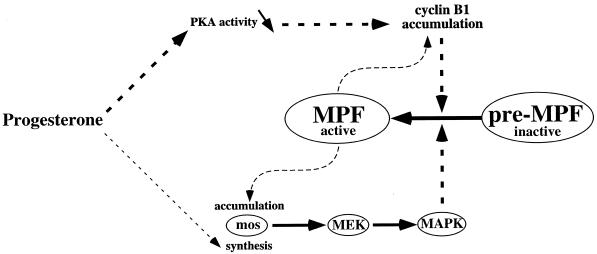
Figure 10.
Model. Cyclin B1 accumulates in response to the decrease of PKA activity induced by progesterone and independently of MPF. Although Mos synthesis has been reported to occur upstream of MPF activation, progesterone does not lead to Mos accumulation unless MPF has been activated.
In contrast to the p34cdc2-dependent accumulation of Mos and activation of the MAPK pathway, we show in this report that the accumulation of cyclin B1 induced by progesterone is independent of p34cdc2 activity. It is also independent of the activation of the MAPK pathway because both Mos accumulation and MAPK activation were blocked by p21cip1. This was confirmed by the injection of mos-specific antisense oligonucleotides into prophase oocytes in the presence of progesterone. Under these conditions and as previously shown (Sagata et al., 1988; Roy et al., 1996), p34cdc2 activation, GVBD, and MAPK activation normally induced by progesterone were inhibited, but the accumulation of cyclin B1 was still observed (our unpublished results). Taken together, these results show that cyclin B1 accumulation occurs in response to progesterone independently of p34cdc2 activity (Figure 10).
The discrepancy between Mos and cyclin B1 protein accumulation is also reflected at the mRNA level. Barkoff et al. (1998) reported that polyadenylation of mos mRNA is not sufficient for accumulation of the protein in the absence of progesterone. Moreover, when meiotic maturation is inhibited by the presence of kinase-inactive p34cdc2 (K33R), mos mRNA is still polyadenylated in response to progesterone (Ballantyne et al., 1997) but the protein does not accumulate (Nebreda et al., 1995). Conversely, under the same conditions, cyclin B1 mRNA does not undergo additional polyadenylation (Ballantyne et al., 1997) but the protein does accumulate (Nebreda et al., 1995). Furthermore, it has been shown that inhibition of cap-ribose methylation by SIBA prevents mos mRNA translation (Kuge et al., 1998); in contrast, our results demonstrate that, unlike Mos, cyclin B1 accumulates normally in the presence of SIBA. Thus, polyadenylation and cap-ribose methylation appear to be necessary but not sufficient for the accumulation of Mos, whereas the level of cyclin B1 can increase in the absence of polyadenylation or cap-ribose methylation of mRNA. Accumulation of cyclin B1 occurs over several hours. Our results with injected cyclin B1 suggest that the accumulation of cyclin B1 induced by progesterone in the presence of p21cip1 does not involve a decreased rate of degradation. However, we cannot rigorously exclude the possibility that a slight change in the balance between synthesis and degradation over several hours could contribute to the accumulation of cyclin B1 induced by progesterone.
One biochemical change known to control Xenopus oocyte maturation is a decrease in the activity of PKA. Maller and Krebs (1977) showed that PKAc inhibits progesterone-induced maturation, whereas PKI and the regulatory subunit of PKA induce meiotic maturation directly in the absence of progesterone. A decrease in cAMP concentration after progesterone addition correlates with the inhibition of PKA and is the first early event known to occur upstream of Mos synthesis and p34cdc2 activation (Speaker and Butcher, 1977; Maller et al., 1979). The results in this paper provide evidence that the accumulation of cyclin B1 is another response of the oocyte to progesterone that is independent of Mos synthesis and p34cdc2 activation. We also analyzed directly the effects of an increase or decrease in PKA activity on cyclin B1 accumulation. Our results demonstrate that inhibition of PKA is sufficient to allow cyclin B1 accumulation when p34cdc2 activation is inhibited by p21cip1. The reciprocal experiment shows that injection of PKAc prevents the accumulation of cyclin B1 induced by progesterone. The hormone, therefore, triggers cyclin B1 accumulation by acting through a decrease in PKA activity (Figure 10). This again differentiates cyclin B1 from Mos in terms of regulation, because it was recently shown that PKA does not exert any inhibitory effect on Mos translation induced by exogenous Mos injection (Faure et al., 1998). However, GVBD does not occur when exogenous Mos is injected in the presence of PKAc (Daar et al., 1993). These results favor the view that accumulation of cyclin B1 may be required for Mos to trigger meiotic maturation.
What could be the functional role of cyclin B1 accumulation? It is well established that microinjection of cyclin B protein induces GVBD in the absence of protein synthesis (Roy et al., 1991). Our findings demonstrate that cyclin B1 accumulates in response to progesterone in the absence of MPF activation. Furthermore, a recent report by de Moor and Richter (1999) shows that the stimulation of endogenous cyclin B1 translation is sufficient to induce meiotic maturation.
Together, these experimental data suggest that cyclin B1 accumulation could be a physiological trigger of preMPF activation in oocytes. This pathway is indeed the shortest link between progesterone and preMPF activation (Figure 10).
Recently, a new cdk2-interacting protein encoded by the spy1 gene was cloned in Xenopus (Lenormand et al., 1999). The spy1 mRNA is capable of inducing MAPK and MPF activation as well as triggering meiotic maturation when injected into prophase oocytes, with a kinetic close to the one observed after cyclin B mRNA microinjection. Although there is no evidence at this time that spy1 protein is present and/or translated in oocytes, this protein represents a new potential player in the transduction pathway leading to MPF activation.
In summary, the accumulation of cyclin B1 represents an early step in the transduction pathway induced by progesterone in the oocyte. It clearly lies downstream of the decrease in PKA activity induced by the hormone. It is significant that this accumulation is independent of the mechanisms that lead to Mos accumulation, including stimulation of cap-ribose methylation. Additional studies on the mechanism of cyclin B1 accumulation should advance our understanding of the signal transduction pathways regulated by progesterone during oocyte maturation.
ACKNOWLEDGMENTS
We are grateful to Eleanor Erikson for critically reading the manuscript. This work was supported by the Centre National de la Recherche Scientifique, the Institut National de la Recherche Agronomique, the Université Pierre et Marie Curie, and by grants from the National Institutes of Health (GM26743 and DK28353). J.L.M. is an investigator of the Howard Hughes Medical Institute.
Abbreviations used:
- GVBD
germinal vesicle breakdown
- MPF
maturation-promoting factor
- OA
okadaic acid
- PKA
cAMP-dependent protein kinase
- PKAc
catalytic subunit of cAMP-dependent protein kinase
- PKI
thermostable inhibitor of the catalytic subunit of cAMP-dependent protein kinase
- SIBA
S-isobutylthioadenosine
REFERENCES
- Anderson CW, Baum PR, Gesteland RF. Processing of adenovirus 2-induced proteins. J Virol. 1973;12:241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne S, Daniel DL, Wickens M. A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and CDK1 activation. Mol Biol Cell. 1997;8:1633–1648. doi: 10.1091/mbc.8.8.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff A, Ballantyne S, Wickens M. Meiotic maturation in Xenopus requires polyadenylation of multiple mRNAs. EMBO J. 1998;17:3168–3175. doi: 10.1093/emboj/17.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatase. Biochem J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MZ, Cooper JA. The beta subunit of CKII negatively regulates Xenopus oocyte maturation. Proc Natl Acad Sci USA. 1997;94:9136–9140. doi: 10.1073/pnas.94.17.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar I, Yew N, Vande Woude GF. Inhibition of mos-induced oocyte maturation by protein kinase A. J Cell Biol. 1993;120:1197–1202. doi: 10.1083/jcb.120.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor CH, Richter JD. Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J. 1999;18:2294–2303. doi: 10.1093/emboj/18.8.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989;56:829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Brizuela L, Beach D, Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Faure S, Morin N, Doree M. Inactivation of protein kinase A is not required for c-mos translation during meiotic maturation of Xenopus oocytes. Oncogene. 1998;17:1215–1221. doi: 10.1038/sj.onc.1202056. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Bhatt RR. Mechanistic studies of the dual phosphorylation of mitogen-activated protein kinase. J Biol Chem. 1997;272:19008–19016. doi: 10.1074/jbc.272.30.19008. [DOI] [PubMed] [Google Scholar]
- Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, Ogawa Y, Sagata N. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno N, Ogawa Y, Iwashita J, Nakajo N, Sagata N. Meiotic cell cycle in Xenopus oocytes is independent of cdk2 kinase. EMBO J. 1997;16:3860–3865. doi: 10.1093/emboj/16.13.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Maller JL. Cyclin B in Xenopus oocytes: implications for the mechanism of pre-MPF activation. EMBO J. 1991;10:177–182. doi: 10.1002/j.1460-2075.1991.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gautier J, Norbury C, Lohka M, Maller J. Purified maturation promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle gene cdc2+ Cell. 1988;54:433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Wu M, Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J, Hermann J, Hendrix P, Ozon R, Merlevede W. Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett. 1989;245:91–94. doi: 10.1016/0014-5793(89)80198-x. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Moriyama K, Matsuda S, Okumura E, Kishimoto T, Kawasaki H, Suzuki K, Yahara I, Sakai H, Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991;10:2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Huchon D, Ozon R, Fischer EH, Demaille JG. The pure inhibitor of cAMP-dependent protein kinase initiates Xenopus laevis meiotic maturation: a 4-step scheme for meiotic maturation. Mol Cell Endocrinol. 1981;22:211–222. doi: 10.1016/0303-7207(81)90092-7. [DOI] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Elimination of Cdc2 phosphorylation sites in the Cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessus C, Rime H, Haccard O, Van Lint J, Goris J, Merlevede W, Ozon R. Tyrosine phosphorylation of p34cdc2 and p42 during meiotic maturation of Xenopus oocyte: antagonistic action of okadaic acid and 6-DMAP. Development. 1991;111:813–820. doi: 10.1242/dev.111.3.813. [DOI] [PubMed] [Google Scholar]
- Jessus C, Thibier C, Ozon R. Levels of microtubules during the meiotic maturation of the Xenopus oocyte. J Cell Sci. 1987;87:705–712. doi: 10.1242/jcs.87.5.705. [DOI] [PubMed] [Google Scholar]
- Karaiskou A, Cayla X, Haccard O, Jessus C, Ozon R. MPF amplification in Xenopus oocyte extracts depends on a two-step activation of Cdc25 phosphatase. Exp Cell Res. 1998;244:491–500. doi: 10.1006/excr.1998.4220. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Minshull J, Ford C, Golsteyn R, Poon R, Hunt T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol. 1991;114:755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge H, Brownlee GG, Gershon PD, Richter JD. Cap ribose methylation of c-mos mRNA stimulates translation and oocyte maturation in Xenopus laevis. Nucleic Acids Res. 1998;26:3208–3214. doi: 10.1093/nar/26.13.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenormand JL, Dellinger RW, Knudsen KE, Subramani S, Donoghue DJ. Speedy: a novel cell cycle regulator of the G2/M transition. EMBO J. 1999;18:1869–1877. doi: 10.1093/emboj/18.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JL, Butcher FR, Krebs EG. Early effect of progesterone on levels of cyclic adenosine 3′:5′-monophosphate in Xenopus oocytes. J Biol Chem. 1979;254:579–582. [PubMed] [Google Scholar]
- Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes: induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1977;252:1712–1718. [PubMed] [Google Scholar]
- Masui Y, Clarke H. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–146. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Kosako H, Takenaka K, Moriyama K, Sakai H, Akiyama T, Gotoh Y, Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992;11:973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Arion D, Golsteyn R, Pines J, Brizuela L, Hunt T, Beach D. Cyclin is a component of sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989;8:2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda A, Gannon J, Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 1995;14:5597–5607. doi: 10.1002/j.1460-2075.1995.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Hill C, Gomez N, Cohen P, Hunt T. The protein kinase mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett. 1993;333:183–187. doi: 10.1016/0014-5793(93)80401-f. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Furuno N, Okazaki K, Tanaka H, Ogawa Y, Sagata N. Degradation of MOS by the N-terminal proline (Pro(2))-dependent ubiquitin pathway on fertilization of Xenopus eggs: possible significance of natural selection for Pro(2) in Mos. EMBO J. 1993;12:4021–4027. doi: 10.1002/j.1460-2075.1993.tb06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted JB. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981;256:11955–11957. [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn NG, Vande Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel RE, Sleight SB, Maller JL. Maternal Xenopus Cdk2-cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J Biol Chem. 1995;270:6843–6855. doi: 10.1074/jbc.270.12.6843. [DOI] [PubMed] [Google Scholar]
- Rime H, Huchon D, Jessus C, Goris J, Merlevede W, Ozon R. Characterization of MPF activation by okadaic acid in Xenopus oocyte. Cell Differ Dev. 1990;29:47–58. doi: 10.1016/0922-3371(90)90023-p. [DOI] [PubMed] [Google Scholar]
- Roy LM, Haccard O, Izumi T, Lattes BG, Lewellyn AL, Maller JL. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- Roy LM, Swenson KI, Walker DH, Gabrielli BG, Li RS, Piwnica-Worms H, Maller JL. Activation of p34cdc2 kinase by cyclin A. J Cell Biol. 1991;113:507–514. doi: 10.1083/jcb.113.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N, Daar I, Oskarsson M, Showalter SD, Vande Woude GF. The product of the mos proto-oncogene as a candidate “initiator” for oocyte maturation. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Shibuya E, Ruderman J. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speaker MG, Butcher FR. Cyclic nucleotide fluctuations during steroid induced meiotic maturation of frog oocytes. Nature. 1977;267:848–850. doi: 10.1038/267848a0. [DOI] [PubMed] [Google Scholar]
- Su J, Rempel R, Erikson E, Maller J. Cloning and characterization of the Xenopus cyclin-dependent kinase inhibitor p27XIC1. Proc Natl Acad Sci USA. 1995;92:10187–10191. doi: 10.1073/pnas.92.22.10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibier C, De Smedt V, Poulhe R, Huchon D, Jessus C, Ozon R. In vivo regulation of cytostatic activity in Xenopus metaphase II-arrested oocytes. Dev Biol. 1997;185:55–66. doi: 10.1006/dbio.1997.8543. [DOI] [PubMed] [Google Scholar]
- Wasserman WJ, Masui Y. Effects of cycloheximide on a cytoplasmic factor initiating meiotic maturation in Xenopus oocytes. Exp Cell Res. 1975;91:381–388. doi: 10.1016/0014-4827(75)90118-4. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]