Abstract
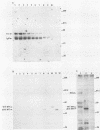
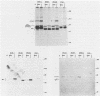
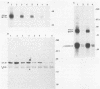
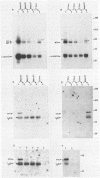
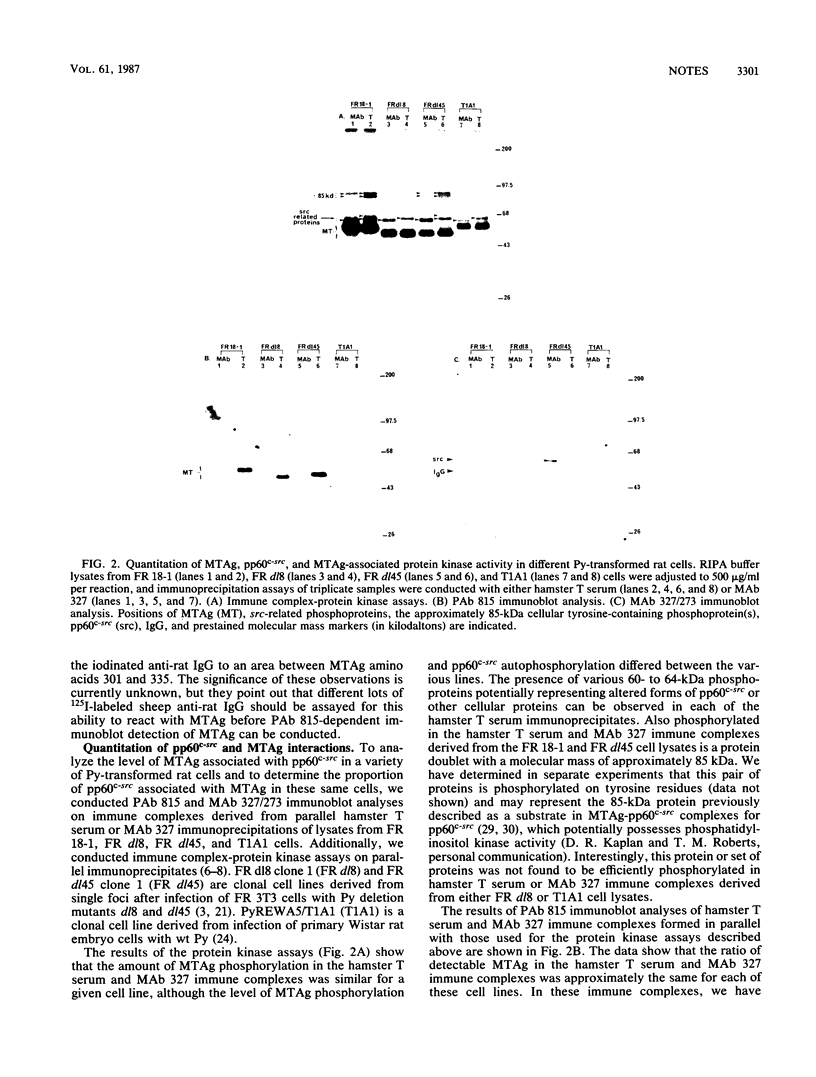
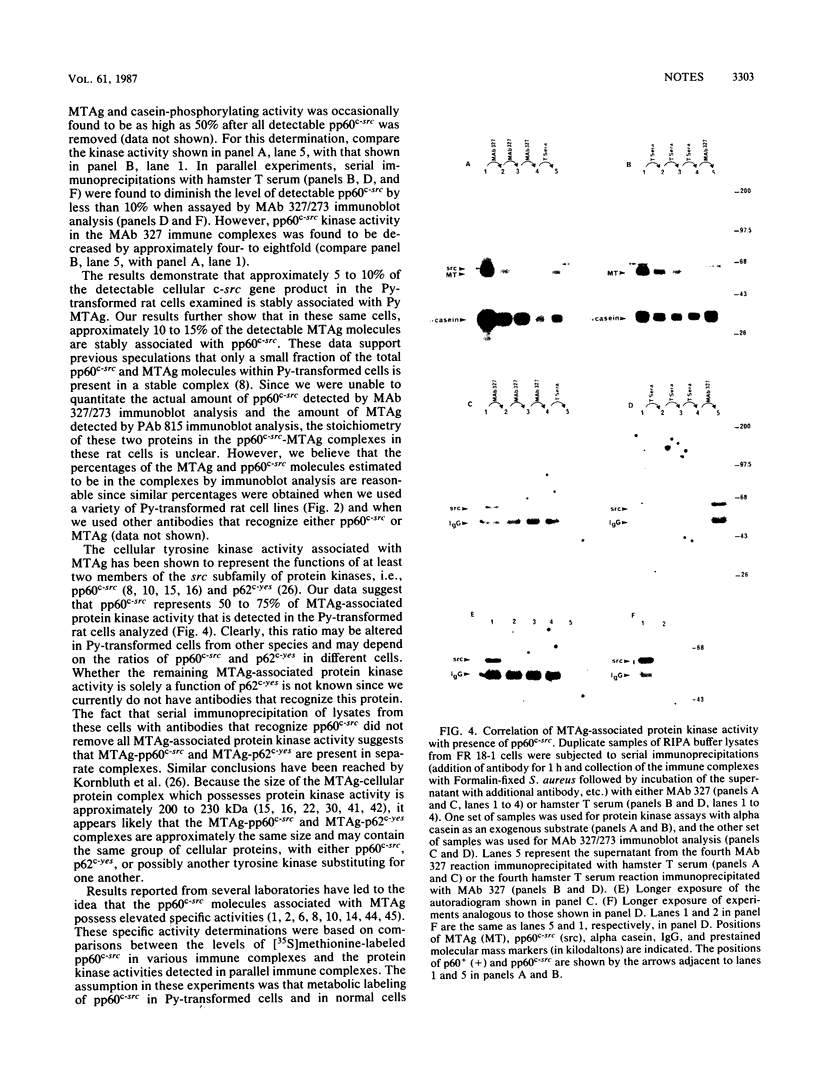
The relative abundance of pp60c-src molecules associated with polyomavirus (Py) middle tumor antigen (MTAg) and the relative abundance of MTAg associated with pp60c-src in a variety of Py-transformed rat cells was determined by quantitative immunoblot analyses which detect pp60c-src or Py MTAg. The results demonstrate that approximately 5 to 10% of the total immunoprecipitable pp60c-src molecules in Py-transformed rat cells are stably associated with MTAg and have elevated protein kinase activities. In these same cells, it was found that approximately 10 to 15% of the detectable MTAg molecules are stably associated with pp60c-src. Other results presented in this report demonstrate that approximately 50 to 75% of the total MTAg-associated cellular tyrosine kinase activity potentially represents the enzymatic activity of pp60c-src, while the remaining 25 to 50% represents the activity of other cellular tyrosine kinases. Our results also show that most pp60c-src molecules associated with Py MTAg do not possess electrophoretic mobilities that are altered from those of pp60c-src molecules not associated with MTAg or pp60c-src molecules obtained from normal rodent cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amini S., DeSeau V., Reddy S., Shalloway D., Bolen J. B. Regulation of pp60c-src synthesis by inducible RNA complementary to c-src mRNA in polyomavirus-transformed rat cells. Mol Cell Biol. 1986 Jul;6(7):2305–2316. doi: 10.1128/mcb.6.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini S., Lewis A. M., Jr, Israel M. A., Butel J. S., Bolen J. B. Analysis of pp60c-src protein kinase activity in hamster embryo cells transformed by simian virus 40, human adenoviruses, and bovine papillomavirus 1. J Virol. 1986 Jan;57(1):357–361. doi: 10.1128/jvi.57.1.357-361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendig M. M., Thomas T., Folk W. R. Viable deletion mutant in the medium and large T-antigen-coding sequences of the polyoma virus genome. J Virol. 1980 Mar;33(3):1215–1220. doi: 10.1128/jvi.33.3.1215-1220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Amini S., DeSeau V., Reddy S., Shalloway D. Analysis of polyomavirus middle-T-antigen-transformed rat cell variants expressing different levels of pp60c-src. J Virol. 1987 Apr;61(4):1079–1085. doi: 10.1128/jvi.61.4.1079-1085.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Israel M. A. Middle tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with pp60c-src. J Virol. 1985 Jan;53(1):114–119. doi: 10.1128/jvi.53.1.114-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Lewis A. M., Jr, Israel M. A. Stimulation of pp60c-src tyrosyl kinase activity in polyoma virus-infected mouse cells is closely associated with polyoma middle tumor antigen synthesis. J Cell Biochem. 1985;27(2):157–167. doi: 10.1002/jcb.240270209. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Rosen N., Israel M. A. Increased pp60c-src tyrosyl kinase activity in human neuroblastomas is associated with amino-terminal tyrosine phosphorylation of the src gene product. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7275–7279. doi: 10.1073/pnas.82.21.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., Deseau V., Rosen N. Analysis of pp60c-src in human colon carcinoma and normal human colon mucosal cells. Oncogene Res. 1987 Jul;1(2):149–168. [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Hutchinson M. A., Eckhart W. Structural and functional modification of pp60c-src associated with polyoma middle tumor antigen from infected or transformed cells. Mol Cell Biol. 1985 Oct;5(10):2647–2652. doi: 10.1128/mcb.5.10.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Markland W., Markham A. F., Smith A. E. Mutations around the NG59 lesion indicate an active association of polyoma virus middle-T antigen with pp60c-src is required for cell transformation. EMBO J. 1986 Feb;5(2):325–334. doi: 10.1002/j.1460-2075.1986.tb04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D., Hassell J. A. Overproduction of polyomavirus middle T antigen in mammalian cells through the use of an adenovirus vector. J Virol. 1987 Apr;61(4):1226–1239. doi: 10.1128/jvi.61.4.1226-1239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Ito Y., Novak U., Spurr N., Dilworth S., Smolar N., Pollack R., Smith K., Rifkin D. B. Early mutants of polyoma virus (dl8 and dl23) with altered transformation properties: is polyoma virus middle T antigen a transforming gene product? Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):271–283. doi: 10.1101/sqb.1980.044.01.031. [DOI] [PubMed] [Google Scholar]
- Grussenmeyer T., Scheidtmann K. H., Hutchinson M. A., Eckhart W., Walter G. Complexes of polyoma virus medium T antigen and cellular proteins. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7952–7954. doi: 10.1073/pnas.82.23.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Brocklehurst J. R., Dulbecco R. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by pol-oma virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4666–4670. doi: 10.1073/pnas.74.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Sudol M., Hanafusa H. Association of the polyomavirus middle-T antigen with c-yes protein. Nature. 1987 Jan 8;325(7000):171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Cheng S. H., Oostra B. A., Smith A. E. In vitro mutagenesis of the putative membrane-binding domain of polyomavirus middle-T antigen. J Virol. 1986 Jul;59(1):82–89. doi: 10.1128/jvi.59.1.82-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Smith A. E. Mapping of the amino-terminal half of polyomavirus middle-T antigen indicates that this region is the binding domain for pp60c-src. J Virol. 1987 Feb;61(2):285–292. doi: 10.1128/jvi.61.2.285-292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. T., Benjamin T. L. 12-O-tetradecanoylphorbol-13-acetate stimulates phosphorylation of the 58,000-Mr form of polyomavirus middle T antigen in vivo: implications for a possible role of protein kinase C in middle T function. J Virol. 1986 May;58(2):239–246. doi: 10.1128/jvi.58.2.239-246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. V., Tyndall C., Magnusson G. Deletion mapping of a short polyoma virus middle T antigen segment important for transformation. J Virol. 1983 Apr;46(1):284–287. doi: 10.1128/jvi.46.1.284-287.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis L., Lamfrom H., Benjamin T. L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen N., Bolen J. B., Schwartz A. M., Cohen P., DeSeau V., Israel M. A. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986 Oct 15;261(29):13754–13759. [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Bockus B. J., Berkner K. L., Kaplan D., Roberts T. M. Characterization of middle T antigen expressed by using an adenovirus expression system. J Virol. 1987 Apr;61(4):1221–1225. doi: 10.1128/jvi.61.4.1221-1225.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Templeton D., Eckhart W. Mutation causing premature termination of the polyoma virus medium T antigen blocks cell transformation. J Virol. 1982 Mar;41(3):1014–1024. doi: 10.1128/jvi.41.3.1014-1024.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton D., Voronova A., Eckhart W. Construction and expression of a recombinant DNA gene encoding a polyomavirus middle-size tumor antigen with the carboxyl terminus of the vesicular stomatitis virus glycoprotein G. Mol Cell Biol. 1984 Feb;4(2):282–289. doi: 10.1128/mcb.4.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Walter G., Carbone A., Welch W. J. Medium tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with 73-kilodalton heat shock protein. J Virol. 1987 Feb;61(2):405–410. doi: 10.1128/jvi.61.2.405-410.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Purification of polyoma virus medium-size tumor antigen by immunoaffinity chromatography. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4025–4029. doi: 10.1073/pnas.79.13.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Yonemoto W., Jarvis-Morar M., Brugge J. S., Bolen J. B., Israel M. A. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4568–4572. doi: 10.1073/pnas.82.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]