Abstract
In order to determine the distribution and prevalence of human cryptosporidiosis on western and southern coastal islands of Jeollanam-do (Province), fecal samples were collected from 2,541 people residing on 25 islands, 13 in the western coasts and 12 in the southern coasts, during July and August 2000. Fecal smears were prepared following formalin-ether sedimentation of the samples and stained by a modified acid-fast procedure. The presence of Cryptosporidium oocysts was determined by light microscopy. Cryptosporidium oocysts were detected in 38 specimens (1.5%). The oocyst positive rate varied (0-6.0%) according to island; the highest was detected on Oenarodo (6.0%), followed by Naenarodo (5.6%) and Nakwoldo (5.4%). The majority (35 persons, 94.6%) of Cryptosporidium-infected individuals were older than 50 years of age. Men (22/1,159; 1.9%) were infected at a higher rate than women (16/1,382; 1.2%). The results of the present survey indicate that human Cryptosporidium infections (due to Cryptosporidium hominis and/or C. parvum) are maintained at a relatively low prevalence on coastal islands of Jeollanam-do, Republic of Korea.
Keywords: Cryptosporidium hominis, Cryptosporidium parvum, cryptosporidiosis, prevalence, coastal islands, Jeollanam-do (Province)
Cryptosporidium is a small coccidian protozoan which infects mammals, birds, reptiles, and fishes. The agents responsible for the majority of human infections are Cryptosporidium hominis (strong anthroponotic transmission) and Cryptosporidium parvum (zoonotic transmission) (Morgan-Ryan et al., 2002;Park et al., 2006).
In the Republic of Korea, after the first confirmation of the presence of C. parvum in laboratory mice (Chai et al., 1990), high prevalences (according to detection methods: 10-22%) of human cryptosporidiosis were detected among out-patients at the Severance Hospital (Cho et al., 1993). Thereafter, cryptosporidiosis has been regarded as an important disease from the public health aspect in the Republic of Korea. Subsequently, several investigators have reported variable prevalences in differing localities (Chai et al., 1996; Seo et al., 2001; Yu et al., 2004; Park et al., 2006). In particular, epidemiological studies on human cryptosporidiosis in Jeollanam-do uncovered unique epidemiological characteristics, including a predominance of infection among aged people, caused by both C. hominis and C. parvum, and animal infections with C. hominis (Chai et al., 2001; Park et al., 2006). Cryptosporidiosis can persist for extended periods of time on coastal islands, because immunocompetent humans infected with Cryptosporidium tend to exhibit only mild symptoms, including self-limiting diarrhea, thus can play a role of the reservoir likewise domestic animals (Okhuysen et al., 1999; Chai et al., 2001).
There are a total of 278 human-inhabited islands in Jeollanam-do (Province). The majority of small coastal islands are both geographically and socio-economically isolated from the mainland. The provision of sufficient medical services for the people on coastal islands has proved difficult, and the residents may have somewhat peculiar life patterns, eating different foods, doing different works, and inhabiting in a different environment from that of the mainland. Therefore, patterns of parasitic infections on coastal islands may also differ from inland patterns. For example, intestinal fluke infections caused by brackish water fishes or bivalves tend to prevail among people living on western and southern coastal islands (Chai and Lee, 2002). However, only a little information is available on the prevalence of intestinal protozoa on coastal islands. A relatively low prevalence (5.5%) of intestinal protozoa, including Entamoeba histolytica, Giardia lamblia, Entamoeba coli, and Endolimax nana, has been reported on the coastal island, Yondo, which is located within the southern regions of Jeollanam-do (Goo et al., 1988). However, no information is currently available regarding Cryptosporidium infections on coastal islands. The present study was conducted in order to characterize the distribution and prevalence of human cryptosporidiosis occurring on coastal islands of Jeollanam-do, Republic of Korea.
Among 278 inhabited islands located off the western and southern coasts of Jeollanam-do, 13 western and 12 southern islands (Fig. 1), on which more than 100 people resided, were randomly selected and epidemiological surveys were conducted. Fecal samples were collected from 2,541 of the islanders during July and August 2000, with the help of the branch offices of the Korea Association of Health Promotion. Fecal examinations were conducted by the formalin ether concentration technique. The fecal sediments were smeared onto glass slides and stained by a modified acid-fast procedure (Chai et al., 2001). The presence of Cryptosporidium oocysts, which are 4-5 µm in diameter, harboring 4 sporozoites, and stained red on a blue background, was visualized under the oil immersion lens of a light microscope. The statistical significance of prevalences, in accordance with geographical location, age, and sex, were analyzed by the chi-square test. Values of P < 0.05 were considered to be significant.
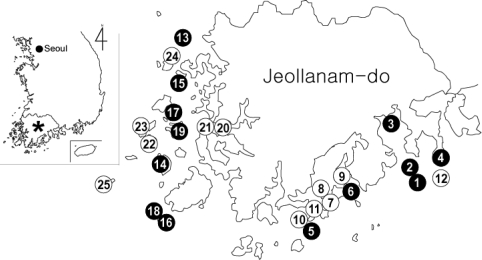
Fig. 1.
Map showing the surveyed islands and the distribution of cryptosporidiosis. The islands are illustrated with numbers, in accordance with Table 1. Islands with Cryptosporidium oocyst positive cases are in black circles and those without positive cases are shown in white circles.
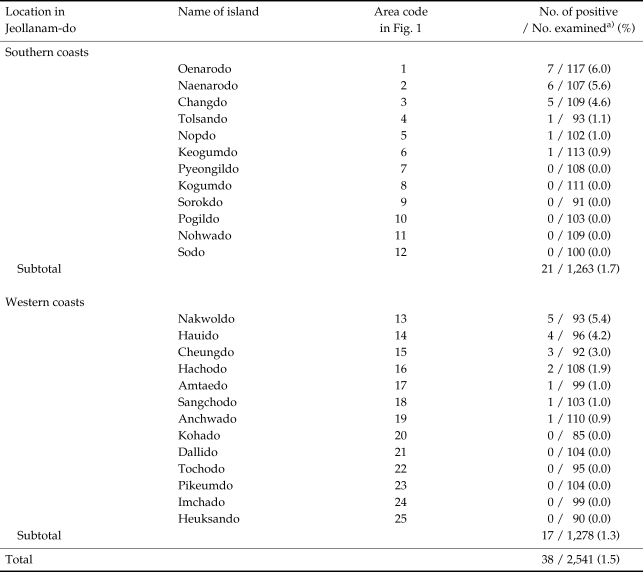
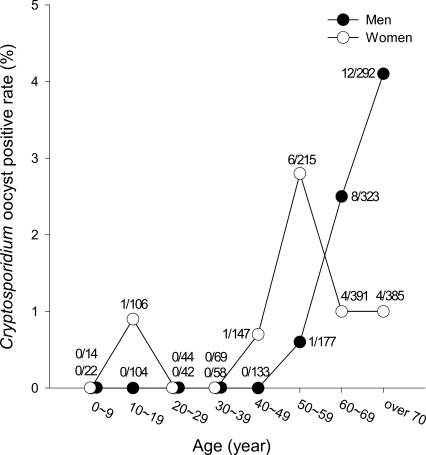
Cryptosporidium oocysts were detected in 38 (1.5%) of 2,541 fecal specimens (Table 1; Fig. 1). On 13 islands which showed oocyst positive cases, the average oocyst positive rate was 2.8%. These islands were scattered along the western and southern coasts, with no special predilection for locality. Of the 12 islands located along the southern coasts, Cryptosporidium infections were detected on 6 islands, Oenarodo (oocyst positive rate: 6.0%), Naenarodo (5.6%), Changdo (4.6%), Tolsando (1.1%), Nopdo (1.0%), and Keogumdo (0.9%) (Table 1; Fig. 1). The average oocyst positive rate on the 12 southern islands was 1.7% (21/1,263 examined) (Table 1). Similarly, among 13 western islands, Cryptosporidium oocysts were detected on 7 islands, including Nakwoldo (5.4%), Hauido (4.2%), Cheungdo (3.0%), Hachodo (1.9%), Amtaedo (1.0%), Sangchodo (1.0%), and Anchwado (0.9%) (Table 1; Fig. 1). The average oocyst positive rate on the 13 western islands was 1.3% (17/1,278 examined). Among the total of 38 positive individuals, 22 (1.9%) were males and 16 (1.2%) were females (Fig. 2). The majority (94.6%) of the oocyst positive cases were over 50 years of age (P = 0.000) (Fig. 2).
Table 1.
Prevalence of human cryptosporidiosis on 25 southern and western coastal islands of Jeollanam-do (Province), Republic of Korea
a)Examined by formalin-ether sedimentation and modified acid-fast (MAF) stain.
Fig. 2.
The age- and sex-prevalence of cryptosporidiosis among people on the southern and western coastal islands of Jeollanam-do, Korea. Numbers (a/b) for each circle represent the number of oocyst positive cases (a) and the number of residents evaluated (b).
In this epidemiological study, the overall human Cryptosporidium oocyst positive rate on the coastal islands of Jeollanam-do, Korea was lower (1.5%) than expected. Cryptosporidium infections were detected on approximately half of the islands (13 of 25 islands). This relatively low prevalence of Cryptosporidium and the limited distribution on coastal islands of Jeollanam-do are interesting results. In previous studies conducted in inland areas of Jeollanam-do, higher prevalences were reported, 10.6% in 1992 (Chai et al., 1996) and 8.2% in 2002 (Yu et al., 2004). These discrepancies between coastal islands and inland areas and environmental factors.
Most coastal islands are geographically separated from inland areas. Migrations of people, as well as reservoir animals, between coastal islands and inland areas are less frequent than are observed within inland areas. Therefore, Cryptosporidium infection and transmission may be limited within islands.
Contamination of drinking water with oocysts constitutes an important risk factor for high cryptosporidiosis prevalence (Kelly et al., 1997). The islands examined in this study are geographically remote from inland areas, and the residents are unable to access treated water from inland. Thus, drinking water is supplied to the islanders from water reservoirs constructed within each island, or from underground. If water reservoirs were contaminated with Cryptosporidium oocysts, many populations would clearly be exposed to infection with the oocysts (Yamamoto et al., 2000). However, in the present study, we detected generally low cryptosporidiosis prevalence on coastal islands, which may imply a relatively low possibility of water reservoir contamination. Another possibility includes the contamination of underground water. However, this possibility is also quite low, as the contamination of underground water with oocysts is uncommon and, on the majority of coastal islands, available water tends to contain some degree of salinity, which can negatively influence the viability of Cryptosporidium oocysts (El Mansoury et al., 2004).
In cases in which the probability of water contamination is low, contact with animal reservoirs should be considered. Domestic animals, including cattle and goats, represent important reservoir hosts for human cryptosporidiosis, and attendants for these animals are regularly exposed to the risk of cryptosporidiosis (Rahaman et al., 1984). However, it is regrettable that no studies have yet been published regarding the status of cryptosporidiosis in domestic animals on island areas in the Republic of Korea. In the case of inland Jeollanam-do, the Cryptosporidium oocyst positive rate among calves suffering from diarrhea was found to be 14.4% (Wee et al., 1996), and this rate was similar to that of humans (10.6%) occupying the same area (Chai et al., 1996). In another observation, the prevalence of cryptosporidiosis was surprisingly high (94%) among the domestic livestock reared in inland areas of Jeollanam-do (Yu et al., 2004).
Cryptosporidiosis induces mild, self-limiting diarrhea in healthy individuals, but can also cause severe diarrhea in young, elderly, or immunocompromised patients (Okhuysen et al., 1999). In the present study, it is worthy of note that the majority (94.6%) of oocyst positive cases were people over 50 years of age. In the questionnaire study, none of these people was determined to suffer from immunocompromised conditions or debilitating diseases. The majority of these patients complained only of mild degenerative diseases, which are common in subjects of advanced age. A similar predilection with regard to age has been reported in an inland rural village of Hwasun-gun, Jeollanam-do (Chai et al., 2001). This high prevalence among elderly subjects is distinct from other reports in other countries; human cryptosporidiosis appears to predominate in children under 10 years of age (Esteban et al., 1998; Leach et al., 2000). One of the reasons for these differences includes that the majority of people living on the islands surveyed in the present study were adults of over 30 years of age and very few children were living on these islands.
Seasonal variations of human cryptosporidiosis have also been reported (McLauchlin et al., 2000; Chai et al., 2001). In the Republic of Korea, as the result of monthly observations in an inland village of Jeollanam-do, the Cryptosporidium oocyst positive rate was lower during summer than in the spring and autumn seasons (Chai et al., 2001). The present study was conducted during the summer season, from July to August. In order to elucidate the seasonal tendencies on the islands surveyed herein, further studies will be necessary.
Unfortunately, we could not isolate Cryptosporidium DNA, due to the insufficient amount of fecal samples and of oocysts collected from each of the individuals assessed in this study. Therefore, we were unable to analyze the genotype and species of Cryptosporidium present on these islands. Our speculation, although unsupported by evidence, is that the infections may represent a mixed population of C. hominis and C. parvum, as has been previously observed in an inland area of Jeollanam-do (Park et al., 2006).
In conclusion, the status of human cryptosporidiosis on the coastal islands of Jeollanam-do, Republic of Korea, has been studied in this work for the first time. It has been determined that these islands evidence a relatively low and variable prevalence (0-6.0%) of cryptosporidiosis. The species of Cryptosporidium involved, i.e., C. hominis, C. parvum, or both, transmission modes, and other epidemiological characteristics of cryptosporidiosis on these islands remain to be studied in the future.
ACKNOWLEDGMENTS
The authors are indebted to members of the headquarters and branch offices of the Korea Association of Health Promotion for their assistance in the collection of the fecal specimens.
References
- 1.Chai JY, Shin SM, Yun CK, Yu JR, Lee SH. Experimental activation of cryptosporidiosis in mice by immunosuppression. Korean J Parasitol. 1990;28:31–37. doi: 10.3347/kjp.1990.28.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Chai JY, Lee SH, Guk SM, Lee SH. An epidemiological survey of Cryptosporidium parvum infection in randomly selected inhabitants of Seoul and Chollanam-do. Korean J Parasitol. 1996;34:113–119. doi: 10.3347/kjp.1996.34.2.113. [DOI] [PubMed] [Google Scholar]
- 3.Chai JY, Kim NY, Guk SM, Park YK, Seo M, Han ET, Lee SH. High prevalence and seasonality of cryptosporidiosis in a small rural village occupied predominantly by aged people in the Republic of Korea. Am J Trop Med Hyg. 2001;65:518–522. doi: 10.4269/ajtmh.2001.65.518. [DOI] [PubMed] [Google Scholar]
- 4.Chai JY, Lee SH. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int. 2002;51:129–154. doi: 10.1016/s1383-5769(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 5.Cho MH, Kim AK, Im KI. Detection of Cryptosporidium oocysts from out-patients of the Severance Hospital, Korea. Korean J Parasitol. 1993;31:193–199. doi: 10.3347/kjp.1993.31.3.193. [DOI] [PubMed] [Google Scholar]
- 6.El Mansoury ST, Abou El Naga IF, Negm AY, Amer EE. Influence of temperature and salinity on the viability and infectivity of Giardia lamblia and Cryptosporidium parvum. J Egypt Soc Parasitol. 2004;34:161–172. [PubMed] [Google Scholar]
- 7.Esteban JG, Aguirre C, Flores A, Strauss W, Angles R, Mas-Coma S. High Cryptosporidium prevalences in healthy Aymara children from the northern Bolivian Altiplano. Am J Trop Med Hyg. 1998;58:50–55. doi: 10.4269/ajtmh.1998.58.50. [DOI] [PubMed] [Google Scholar]
- 8.Goo GS, Min DY, Ahn MH, Kim KM, Leem MH, Yoon HS. Status of intestinal parasitic infections in a remote island, Yondo, Jeonranam-do: province. Korean J Parasitol. 1988;26:275–284. doi: 10.3347/kjp.1988.26.4.275. [DOI] [PubMed] [Google Scholar]
- 9.Kelly P, Baboo KS, Ndubani P, Nchito M, Okeowo NP, Luo NP, Feldman RA, Farthing MJ. Cryptosporidiosis in adults in Lusaka, Zambia, and its relationship to oocyst contamination of drinking water. J Infect Dis. 1997;176:1120–1123. doi: 10.1086/516528. [DOI] [PubMed] [Google Scholar]
- 10.Leach CT, Koo FC, Kuhls TL, Hilsenbeck SG, Jenson HB. Prevalence of Cryptosporidium parvum infection in children along the Texas-Mexico border and associated risk factors. Am J Trop Med Hyg. 2000;62:656–661. doi: 10.4269/ajtmh.2000.62.656. [DOI] [PubMed] [Google Scholar]
- 11.McLauchlin J, Amar C, Pedraza-Diaz S, Nichols GL. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal samples from humans and 105 fecal samples from livestock animals. J Clin Microbiol. 2000;38:3984–3990. doi: 10.1128/jcm.38.11.3984-3990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan-Ryan UM, Fall A, Ward LA, Hijjawi N, Sulaiman I, Fayer R, Thompson RC, Olson M, Lal A, Xiao L. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J Eukaryot Microbiol. 2002;49:433–440. doi: 10.1111/j.1550-7408.2002.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 13.Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Guk SM, Han ET, Shin EH, Kim JL, Chai JY. Genotype analysis of Cryptosporidium spp. prevalent in a rural village in Hwasun-gun, Republic of Korea. Korean J Parasitol. 2006;44:27–33. doi: 10.3347/kjp.2006.44.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahaman AS, Sanyal SC, Al-Mahmud KA, Sobhan A, Hossain KS, Anderson BC. Cryptosporidiosis in calves and their handlers in Bangladesh. Lancet. 1984;28:221. doi: 10.1016/s0140-6736(84)90501-4. [DOI] [PubMed] [Google Scholar]
- 16.Seo M, Huh S, Chai JY, Yu JR. An epidemiology survey on Cryptosporidium parvum infection of inhabitants in Chorwon-gun, Kangwon-do. Korean J Parasitol. 2001;39:201–203. doi: 10.3347/kjp.2001.39.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wee SH, Joo HD, Kang YB. Evaluation for detection of Cryptosporidium oocysts in diarrheal feces of calves. Korean J Parasitol. 1996;34:121–126. doi: 10.3347/kjp.1996.34.2.121. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto N, Urabe K, Takaoka M, Nakazawa K, Gotoh A, Haga M, Fuchigami H, Kimata I, Iseki M. Outbreak of cryptosporidiosis after contamination of the public water supply in Saitama Prefecture, Japan, in 1996. Kansenshogaku Zasshi. 2000;74:518–526. doi: 10.11150/kansenshogakuzasshi1970.74.518. [DOI] [PubMed] [Google Scholar]
- 19.Yu JR, Lee JK, Seo M, Kim SI, Sohn WM, Huh S, Choi HY, Kim TS. Prevalence of cryptosporidiosis among the villagers and domestic animals in several rural areas of Korea. Korean J Parasitol. 2004;42:1–6. doi: 10.3347/kjp.2004.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]