Abstract
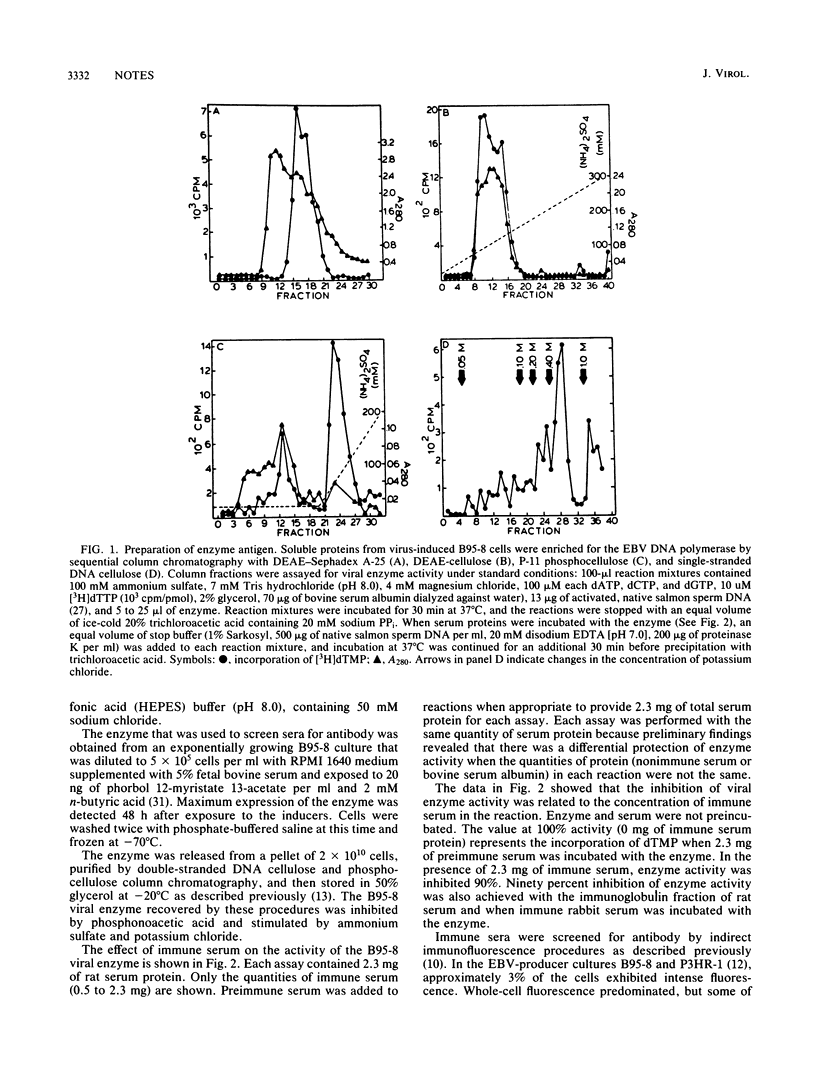
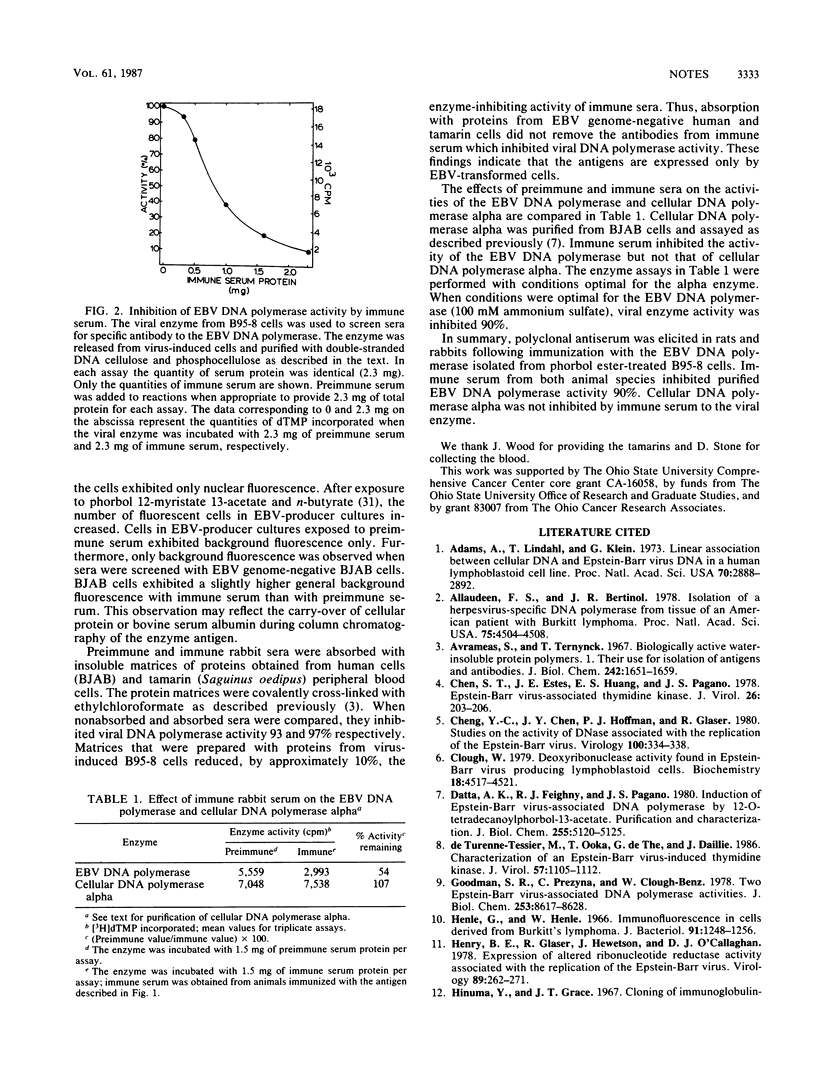
Epstein-Barr virus (EBV) DNA polymerase was released from phorbol ester-treated tamarin (Saguinus oedipus) cells (B95-8) and prepared for use as an antigen by sequential column chromatography with DEAE-Sephadex A-25, DEAE-cellulose, phosphocellulose, and single-stranded DNA cellulose. Proteins from single-stranded DNA cellulose with DNA polymerase activity in 100 mM ammonium sulfate were mixed with complete Freund adjuvant and injected intradermally into rats and rabbits. Immune sera that were screened for specific antibody by indirect immunofluorescence procedures reacted with approximately 3% of the cells in EBV-producer cultures (B95-8 and P3HR-1) but not with EBV genome-negative cells (BJAB). In functional enzyme assays, immune sera or the immunoglobulin fraction inhibited the activity of purified EBV DNA polymerase 90%. Inhibition of enzyme activity was not affected by absorption of immune sera with insoluble matrices of proteins prepared with tamarin and human cells which lacked the EBV genome. Cellular DNA polymerase alpha was not inhibited by immune sera to the EBV enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaudeen H. S., Bertino J. R. Isolation of a herpesvirus-specific DNA polymerase from tissues of an American patient with Burkitt lymphoma. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4504–4508. doi: 10.1073/pnas.75.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Hoffmann P. J., Glaser R. Studies on the activity of DNase associated with the replication of the Epstein-Barr virus. Virology. 1980 Jan 30;100(2):334–338. doi: 10.1016/0042-6822(80)90524-3. [DOI] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- Goodman S. R., Prezyna C., Benz W. C. Two Epstein-Barr virus-associated DNA polymerase activities. J Biol Chem. 1978 Dec 10;253(23):8617–8628. [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. E., Glaser R., Hewetson J., O'Callaghan D. J. Expression of altered ribonucleotide reductase activity associated with the replication of the Epstein-Barr virus. Virology. 1978 Aug;89(1):262–271. doi: 10.1016/0042-6822(78)90058-2. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Kallin B., Sternås L., Saemundssen A. K., Luka J., Jörnvall H., Eriksson B., Tao P. Z., Nilsson M. T., Klein G. Purification of Epstein-Barr virus DNA polymerase from P3HR-1 cells. J Virol. 1985 May;54(2):561–568. doi: 10.1128/jvi.54.2.561-568.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi M., Sugawara K., Ito Y. Epstein-Barr virus-induced early polypeptides in Raji and NC37 cells activated by diterpene ester TPA in combination with N-butyrate. Virology. 1981 Dec;115(2):406–409. doi: 10.1016/0042-6822(81)90123-9. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Mac Gabhann P., Sugawara K., Ito Y. Characterization of Epstein-Barr virus-related thymidine kinase induced in nonproducer cells by superinfection or chemical treatment. Intervirology. 1984;21(2):104–109. doi: 10.1159/000149508. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Heller M., Petti L., O'Shiro E., Kieff E. Persistence of the entire Epstein-Barr virus genome integrated into human lymphocyte DNA. Science. 1984 Dec 14;226(4680):1322–1325. doi: 10.1126/science.6095452. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Glaser R., Rapp F. Studies of an Epstein-Barr virus-induced DNA polymerase. Virology. 1977 Feb;76(2):494–502. doi: 10.1016/0042-6822(77)90232-x. [DOI] [PubMed] [Google Scholar]
- Ooka T., Calender A. Effects of arabinofuranosylthymine on Epstein-Barr virus replication. Virology. 1980 Jul 15;104(1):219–223. doi: 10.1016/0042-6822(80)90379-7. [DOI] [PubMed] [Google Scholar]
- Ooka T., Calender A., de Turenne M., Daillie J. Effect of arabinofuranosylthymine on the replication of Epstein-Barr virus and relationship with a new induced thymidine kinase activity. J Virol. 1983 Apr;46(1):187–195. doi: 10.1128/jvi.46.1.187-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., De Turenne M., De The G., Daillie J. Epstein-Barr virus-specific DNase activity in nonproducer Raji cells after treatment with 12-o-tetradecanoylphorbol-13-acetate and sodium butyrate. J Virol. 1984 Feb;49(2):626–628. doi: 10.1128/jvi.49.2.626-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R., Pendersen M., Kieff E. Complexity of EBV homologous DNA in continous lymphoblastoid cell lines. Virology. 1976 Oct 1;74(1):227–231. [PubMed] [Google Scholar]
- Roubal J., Klein G. Synthesis of thymidine kinase (TK) in Epstein-Barr virus-superinfected Raji TK-negative cells. Intervirology. 1981;15(1):43–48. doi: 10.1159/000149213. [DOI] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Levinger L. F., Carter C. W., Jr Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J Virol. 1979 Feb;29(2):657–665. doi: 10.1128/jvi.29.2.657-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. E. The circular intracellular form of Epstein-Barr virus DNA is amplified by the virus-associated DNA polymerase. J Virol. 1985 Mar;53(3):1012–1015. doi: 10.1128/jvi.53.3.1012-1015.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel P. J., Clough W., Strominger J. L. Sedimentation characteristics of newly synthesized Epstein-Barr viral DNA in superinfected cells. J Virol. 1981 Jun;38(3):880–885. doi: 10.1128/jvi.38.3.880-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R. S., Datta A. K., Cheng Y. C. Identification and characterization of a DNase induced by Epstein-Barr virus. J Virol. 1982 Dec;44(3):893–899. doi: 10.1128/jvi.44.3.893-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. V., Holliday J., Glaser R. Induction of a deoxyuridine triphosphate nucleotidohydrolase activity in Epstein-Barr virus-infected cells. Virology. 1985 Apr 30;142(2):326–333. doi: 10.1016/0042-6822(85)90341-1. [DOI] [PubMed] [Google Scholar]
- de Turenne-Tessier M., Ooka T., de The G., Daillie J. Characterization of an Epstein-Barr virus-induced thymidine kinase. J Virol. 1986 Mar;57(3):1105–1112. doi: 10.1128/jvi.57.3.1105-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]


