Abstract
The effect of neomycin, a phosphoinositide-binding aminoglycoside, on herpes simplex virus type 1 (HSV-1) infection of BHK cells was studied. We showed earlier that it specifically inhibits HSV-1 production but not HSV-2 production (Langeland et al., Biochem Biophys. Res. Commun. 141:198-203, 1986). We now show that neomycin had no effect on cellular protein synthesis, as judged by the appearance of 35S-labeled polypeptides separated by polyacrylamide gel electrophoresis. Virus-induced polypeptides, however, were strongly inhibited at neomycin concentrations above 2 mM. Comparison among different aminoglycosides showed a variation in inhibition of HSV-1 production that paralleled the cationic charge of the aminoglycosides. HSV-1 receptor binding at 4 degrees C was completely inhibited by neomycin. At 37 degrees C both receptor binding and internalization, as measured by an indirect assay, appeared to be inhibited by more than 90%. The effect of neomycin on the infection was almost immediate upon the addition of the drug and preceded virus internalization. Possible mechanisms of the neomycin effect are discussed.
Full text
PDF
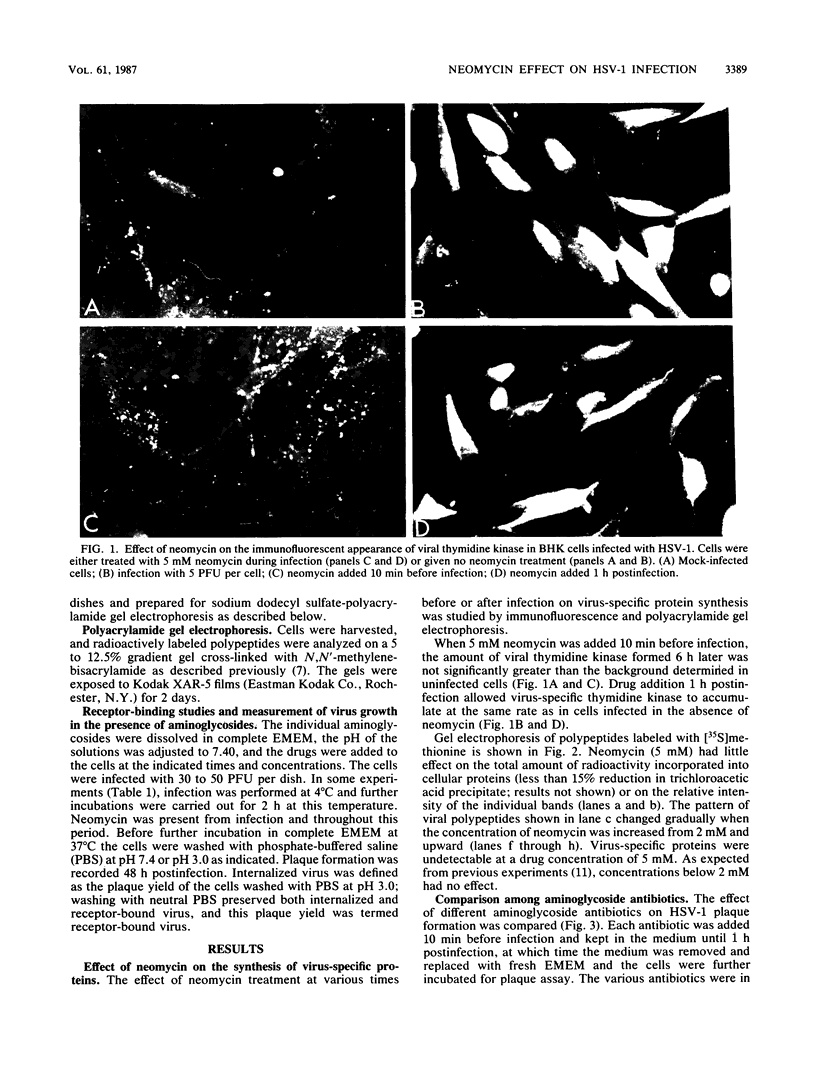
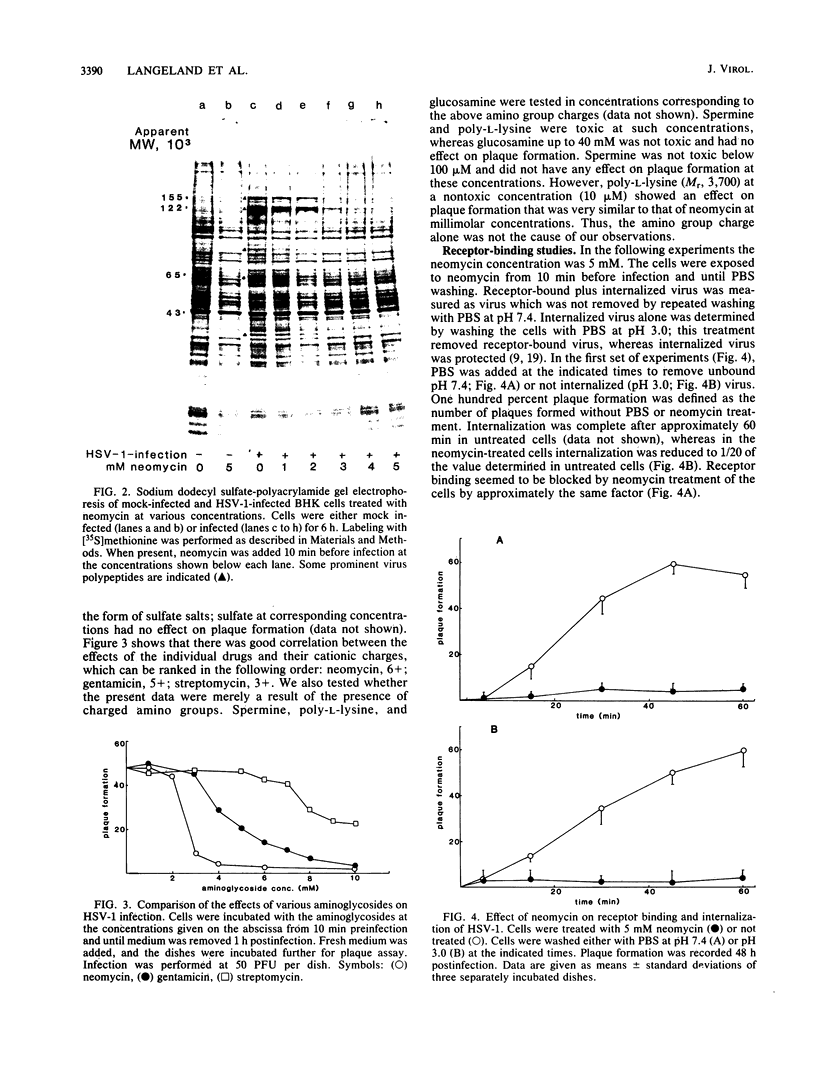
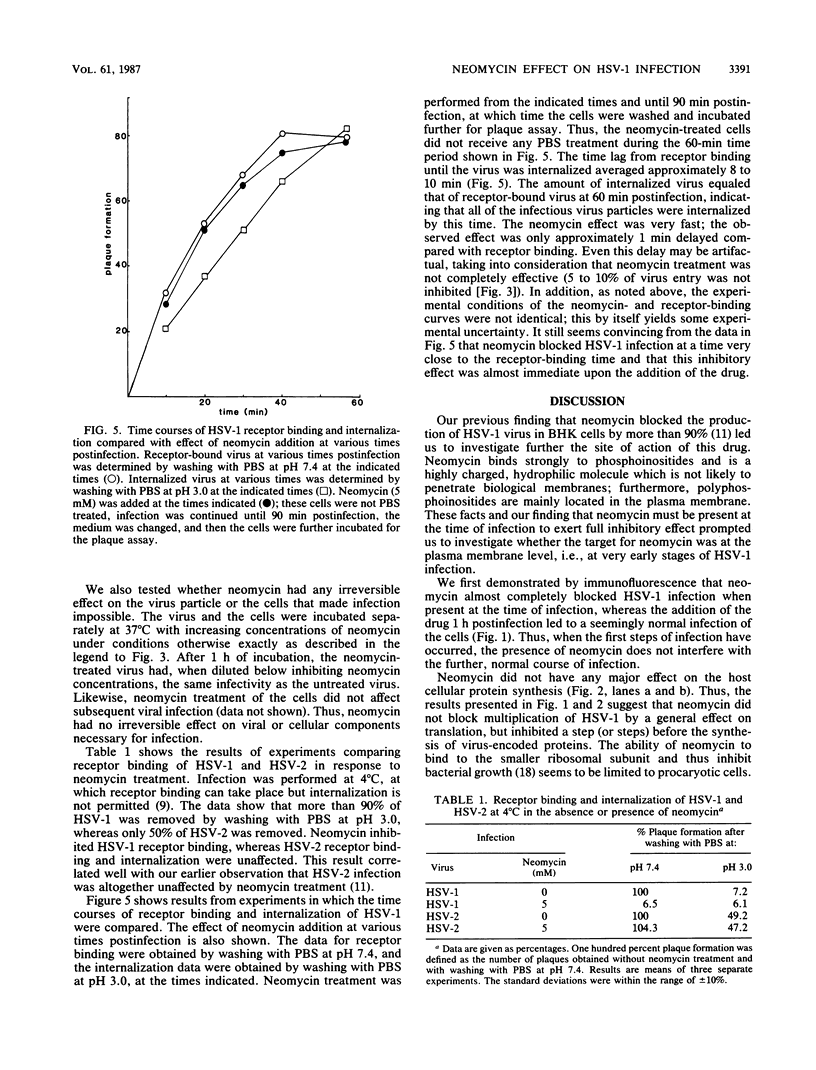


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison C., Rixon F. J., Palfreyman J. W., O'Hara M., Preston V. G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984 Oct 30;138(2):246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Chiu K. C., Westall F., Smith R. A. Covalent linkage of phosphoinositides to myelin basic protein: in vivo occurrence and in vitro studies with experimental allergic encephalomyelitis. Biochem Biophys Res Commun. 1986 Apr 14;136(1):426–432. doi: 10.1016/0006-291x(86)90928-9. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- Haarr L., Marsden H. S., Preston C. M., Smiley J. R., Summers W. C., Summers W. P. Utilization of internal AUG codons for initiation of protein synthesis directed by mRNAs from normal and mutant genes encoding herpes simplex virus-specified thymidine kinase. J Virol. 1985 Nov;56(2):512–519. doi: 10.1128/jvi.56.2.512-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeland N., Haarr L., Holmsen H. Evidence that neomycin inhibits HSV 1 infection of BHK cells. Biochem Biophys Res Commun. 1986 Nov 26;141(1):198–203. doi: 10.1016/s0006-291x(86)80354-0. [DOI] [PubMed] [Google Scholar]
- Langeland N., Haarr L., Holmsen H. Polyphosphoinositide metabolism in baby-hamster kidney cells infected with herpes simplex virus type 1. Biochem J. 1986 Aug 1;237(3):707–712. doi: 10.1042/bj2370707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Marche P., Koutouzov S., Girard A. Impairment of membrane phosphoinositide metabolism by aminoglycoside antibiotics: streptomycin, amikacin, kanamycin, dibekacin, gentamicin and neomycin. J Pharmacol Exp Ther. 1983 Nov;227(2):415–420. [PubMed] [Google Scholar]
- Mohanty J. G., Rosenthal K. S. 2-deoxy-D-glucose inhibition of herpes simplex virus type-1 receptor expression. Antiviral Res. 1986 May;6(3):137–149. doi: 10.1016/0166-3542(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Orsulakova A., Stockhorst E., Schacht J. Effect of neomycin on phosphoinositide labelling and calcium binding in guinea-pig inner ear tissues in vivo and in vitro. J Neurochem. 1976 Feb;26(2):285–290. doi: 10.1111/j.1471-4159.1976.tb04478.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Leuther M. D., Barisas B. G. Herpes simplex virus binding and entry modulate cell surface protein mobility. J Virol. 1984 Mar;49(3):980–983. doi: 10.1128/jvi.49.3.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastrasinh M., Knauss T. C., Weinberg J. M., Humes H. D. Identification of the aminoglycoside binding site in rat renal brush border membranes. J Pharmacol Exp Ther. 1982 Aug;222(2):350–358. [PubMed] [Google Scholar]
- Schacht J. Inhibition by neomycin of polyphosphoinositide turnover in subcellular fractions of guinea-pig cerebral cortex in vitro. J Neurochem. 1976 Nov;27(5):1119–1124. doi: 10.1111/j.1471-4159.1976.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Schibeci A., Schacht J. Action of neomycin on the metabolism of polyphosphoinositides in the guinea pig kidney. Biochem Pharmacol. 1977 Oct 1;26(19):1769–1774. doi: 10.1016/0006-2952(77)90344-6. [DOI] [PubMed] [Google Scholar]
- Stockhorst E., Schacht J. Radioactive labeling of phospholipids and proteins by cochlear perfusion in the guinea pig and the effect of neomycin. Acta Otolaryngol. 1977 May-Jun;83(5-6):401–409. doi: 10.3109/00016487709128864. [DOI] [PubMed] [Google Scholar]
- Timbury M. C. Temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1971 Nov;13(2):373–376. doi: 10.1099/0022-1317-13-2-373. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Svennerholm B., Lycke E. Evidence for herpes simplex virus type-selective receptors on cellular plasma membranes. J Gen Virol. 1979 Jul;44(1):217–225. doi: 10.1099/0022-1317-44-1-217. [DOI] [PubMed] [Google Scholar]
- Van Rooijen L. A., Agranoff B. W. Inhibition of polyphosphoinositide phosphodiesterase by aminoglycoside antibiotics. Neurochem Res. 1985 Aug;10(8):1019–1024. doi: 10.1007/BF00965878. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Aitchison M. Calcium-dependent polyphosphoinositide hydrolysis is associated with exocytosis in vitro. FEBS Lett. 1985 Mar 11;182(1):119–124. doi: 10.1016/0014-5793(85)81167-4. [DOI] [PubMed] [Google Scholar]
- Yang J. C., Chang P. C., Fujitaki J. M., Chiu K. C., Smith R. A. Colvalent linkage of phospholipid to myelin basic protein: identification of phosphatidylinositol bisphosphate as the attached phospholipid. Biochemistry. 1986 May 6;25(9):2677–2681. doi: 10.1021/bi00357a058. [DOI] [PubMed] [Google Scholar]
- Ziegler R. J., Pozos R. S. Effects of lectins on peripheral infections by herpes simplex virus of rat sensory neurons in culture. Infect Immun. 1981 Nov;34(2):588–595. doi: 10.1128/iai.34.2.588-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]