Abstract
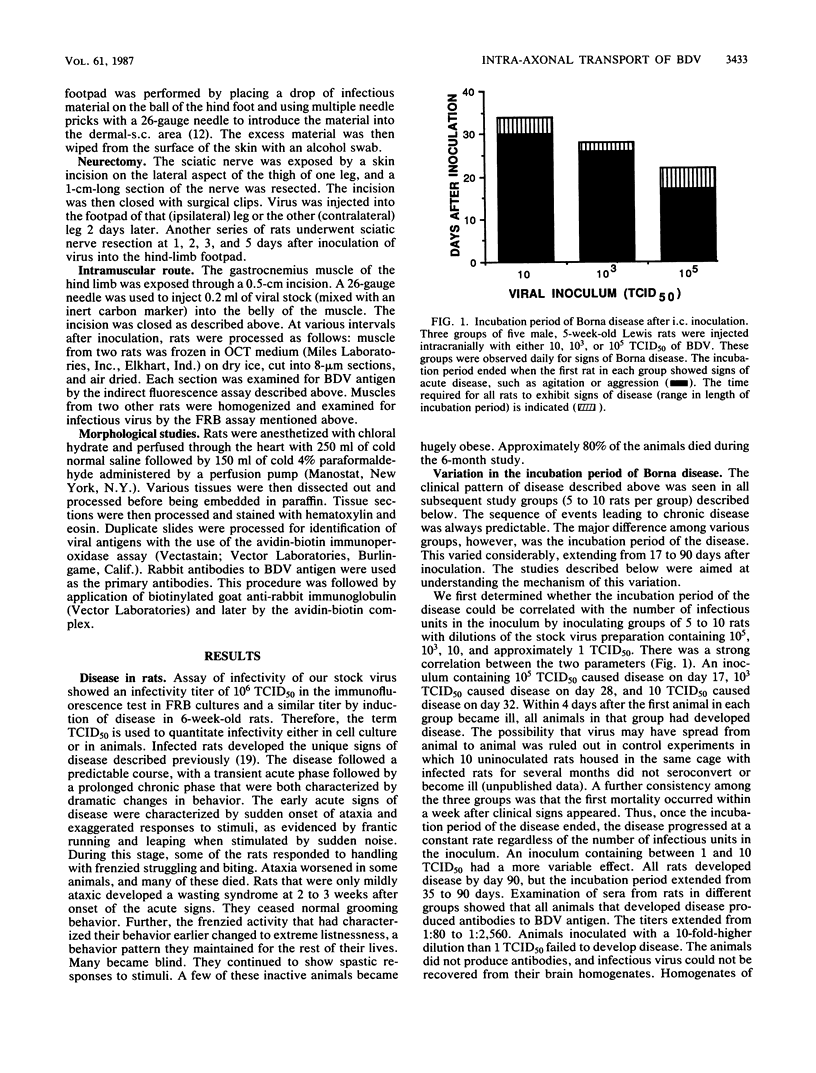
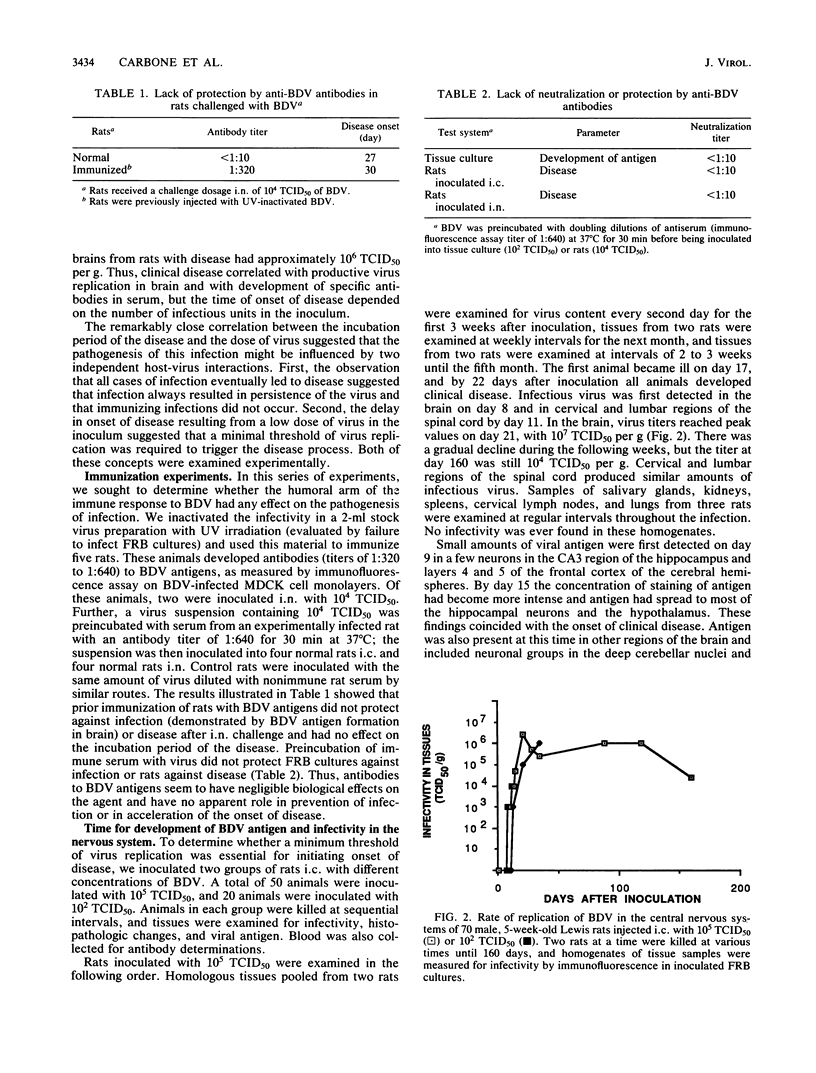
Borna disease virus is an uncharacterized agent that causes sporadic but fatal neurological disease in horses and sheep in Europe. Studies of the infection in rats have shown that the agent has a strict tropism for neural tissues, in which it persists indefinitely. Inoculated rats developed encephalitis after an incubation period of 17 to 90 days. This report shows that the incubation period is the time required for transport of the agent in dendritic-axonal processes from the site of inoculation to the hippocampus. The immune responses to the agent had no effect on replication or transport of the virus. The neural conduit to the brain was proven by intranasal inoculation of virus that resulted in rapid transport of the agent via olfactory nerves to the hippocampus and in development of disease in 20 days. Virus inoculation into the feet resulted in spread along nerve fibers from neuron to neuron. There was sequential replication in neurons of the dorsal root ganglia adjacent to the lumbar spinal cord, the gracilis nucleus in the medulla, and pyramidal cells in the cerebral cortex, followed by infection of the hippocampal neurons and onset of disease. This progression required 50 to 60 days. The exclusiveness of the neural conduit was proven by failure to cause infection after injection of the virus intravenously or into the feet of neurectomized rats.
Full text
PDF


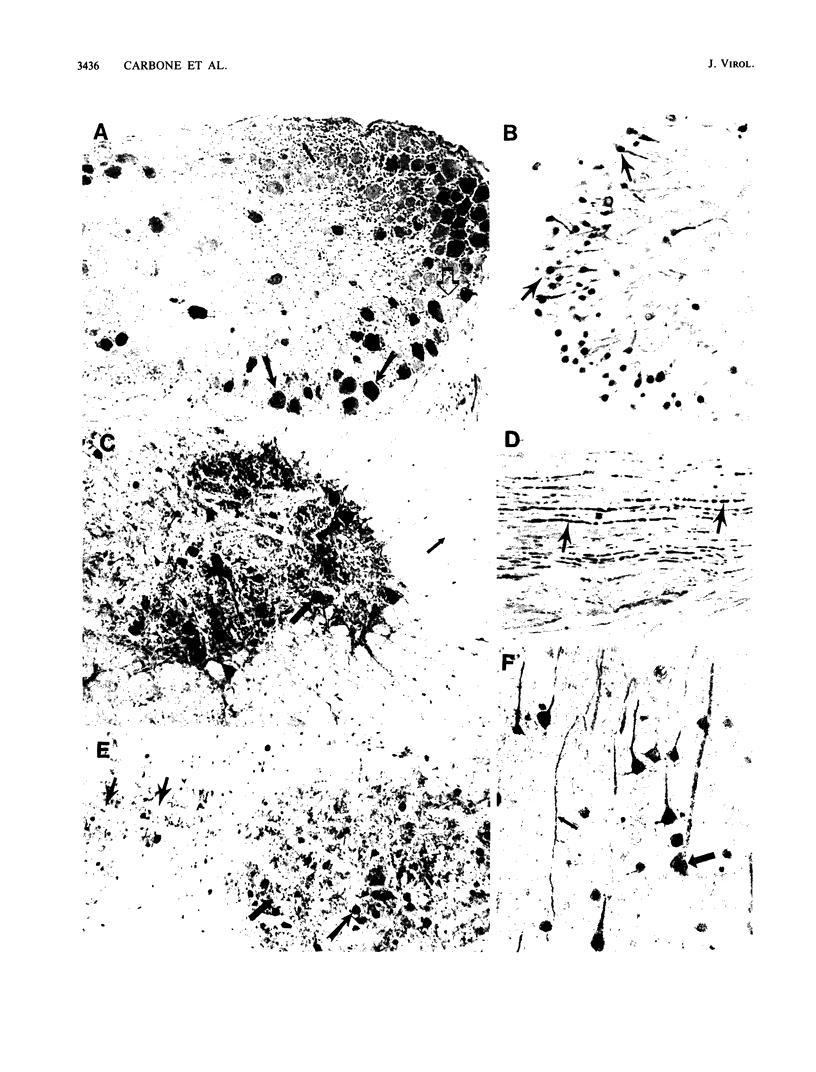



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzil A. P., Blinzinger K. Electron microscopic studies of rabbit central and peripheral nervous system in experimental Borna disease. Acta Neuropathol. 1972;22(4):305–318. doi: 10.1007/BF00809242. [DOI] [PubMed] [Google Scholar]
- Blinzinger K., Anzil A. P. Large granular nuclear bodies (karyosphaeridia) in experimental Borna virus infection. J Comp Pathol. 1973 Oct;83(4):589–596. doi: 10.1016/0021-9975(73)90016-9. [DOI] [PubMed] [Google Scholar]
- Gosztonyi G., Ludwig H. Borna disease of horses. An immunohistological and virological study of naturally infected animals. Acta Neuropathol. 1984;64(3):213–221. doi: 10.1007/BF00688111. [DOI] [PubMed] [Google Scholar]
- Haas B., Becht H., Rott R. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J Gen Virol. 1986 Feb;67(Pt 2):235–241. doi: 10.1099/0022-1317-67-2-235. [DOI] [PubMed] [Google Scholar]
- Herzog S., Kompter C., Frese K., Rott R. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med Microbiol Immunol. 1984;173(4):171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- Herzog S., Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168(3):153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- Hirano N., Kao M., Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J Gen Virol. 1983 Jul;64(Pt 7):1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- Kao M., Ludwig H., Gosztonyi G. Adaptation of Borna disease virus to the mouse. J Gen Virol. 1984 Oct;65(Pt 10):1845–1849. doi: 10.1099/0022-1317-65-10-1845. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol. 1986 Feb;67(Pt 2):255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- Klein R. J., DeStefano E. Dissemination of herpes simplex virus in ganglia after footpad inoculation in neurectomized and non-neurectomized mice. Arch Virol. 1983;77(2-4):231–238. doi: 10.1007/BF01309270. [DOI] [PubMed] [Google Scholar]
- Krey H., Ludwig H., Rott R. Spread of infectious virus along the optic nerve into the retina in Borna disease virus-infected rabbits. Arch Virol. 1979;61(4):283–288. doi: 10.1007/BF01315014. [DOI] [PubMed] [Google Scholar]
- Ludwig H., Koester V., Pauli G., Rott R. The cerebrospinal fluid of rabbits infected with Borna disease virus. Arch Virol. 1977;55(3):209–223. doi: 10.1007/BF01319907. [DOI] [PubMed] [Google Scholar]
- Ludwig H., Kraft W., Kao M., Gosztonyi G., Dahme E., Krey H. Borna-Virus-Infektion (Borna-Krankheit) bei natürlich und experimentell infizierten Tieren: ihre Bedeutung für Forschung und Praxis. Tierarztl Prax. 1985;13(4):421–453. [PubMed] [Google Scholar]
- Ludwig H., Thein P. Demonstration of specific antibodies in the central nervous system of horses naturally infected with Borna disease virus. Med Microbiol Immunol. 1977 Dec 27;163(4):215–226. doi: 10.1007/BF02125505. [DOI] [PubMed] [Google Scholar]
- Mayr A., Danner K. Borna--a slow virus disease. Comp Immunol Microbiol Infect Dis. 1978;1(1-2):3–14. doi: 10.1016/0147-9571(78)90004-8. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna virus in the brain. Science. 1983 Jun 24;220(4604):1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- Roggendorf W., Sasaki S., Ludwig H. Light microscope and immunohistological investigations on the brain of Borna disease virus-infected rabbits. Neuropathol Appl Neurobiol. 1983 Jul-Aug;9(4):287–296. doi: 10.1111/j.1365-2990.1983.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Sprankel H., Richarz K., Ludwig H., Rott R. Behavior alterations in tree shrews (Tupaia glis, Diard 1820) induced by Borna disease virus. Med Microbiol Immunol. 1978 May 26;165(1):1–18. doi: 10.1007/BF02121228. [DOI] [PubMed] [Google Scholar]
- Stitz L., Krey H., Ludwig H. Borna disease in rhesus monkeys as a models for uveo-cerebral symptoms. J Med Virol. 1981;6(4):333–340. doi: 10.1002/jmv.1890060408. [DOI] [PubMed] [Google Scholar]
- Waelchli R. O., Ehrensperger F., Metzler A., Winder C. Borna disease in a sheep. Vet Rec. 1985 Nov 9;117(19):499–500. doi: 10.1136/vr.117.19.499. [DOI] [PubMed] [Google Scholar]