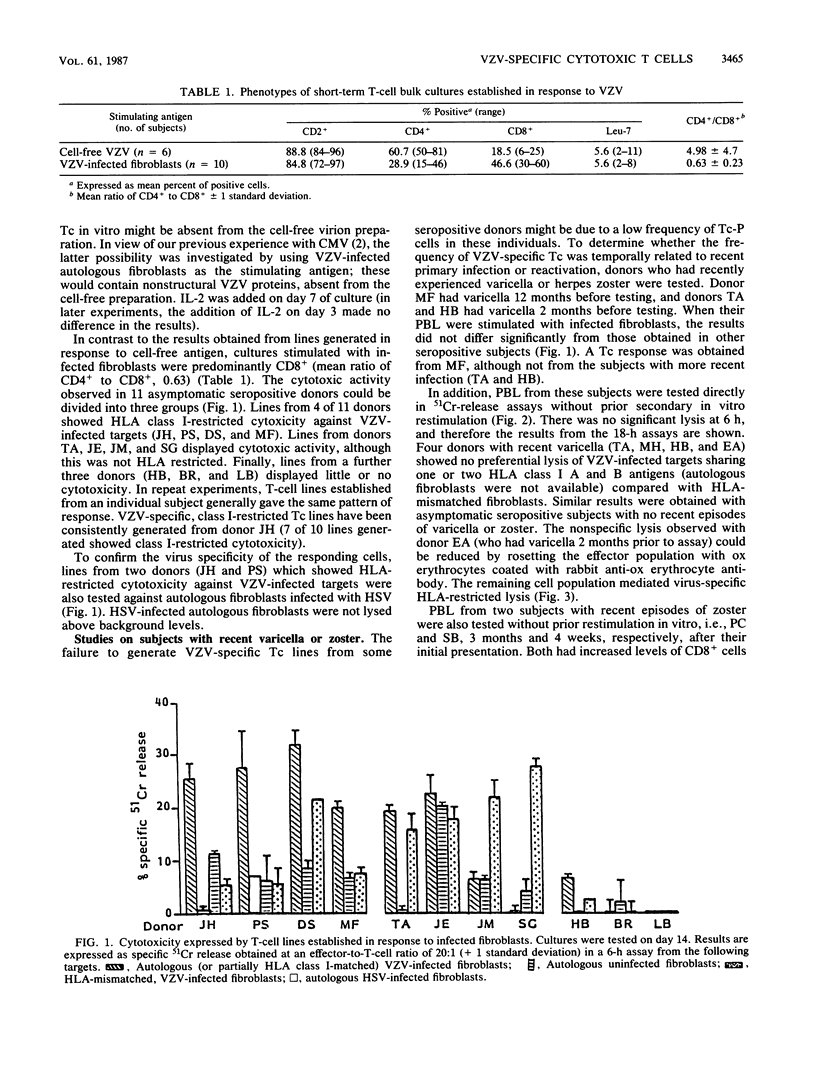
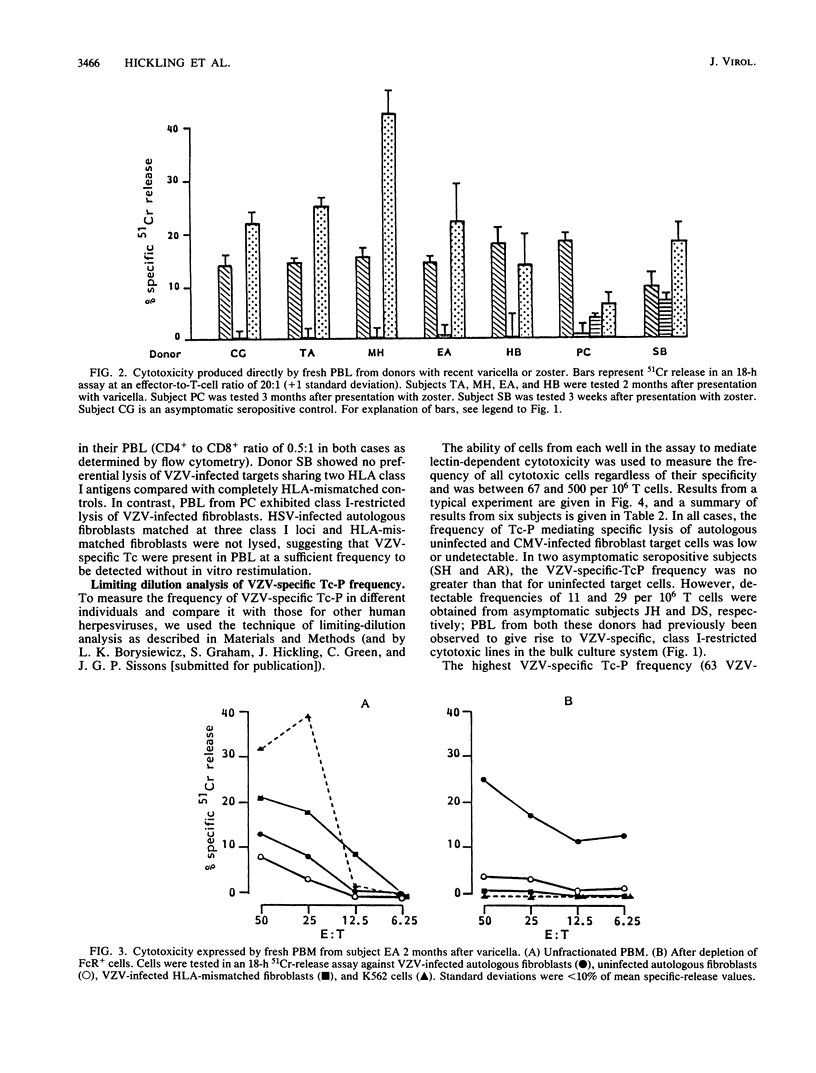
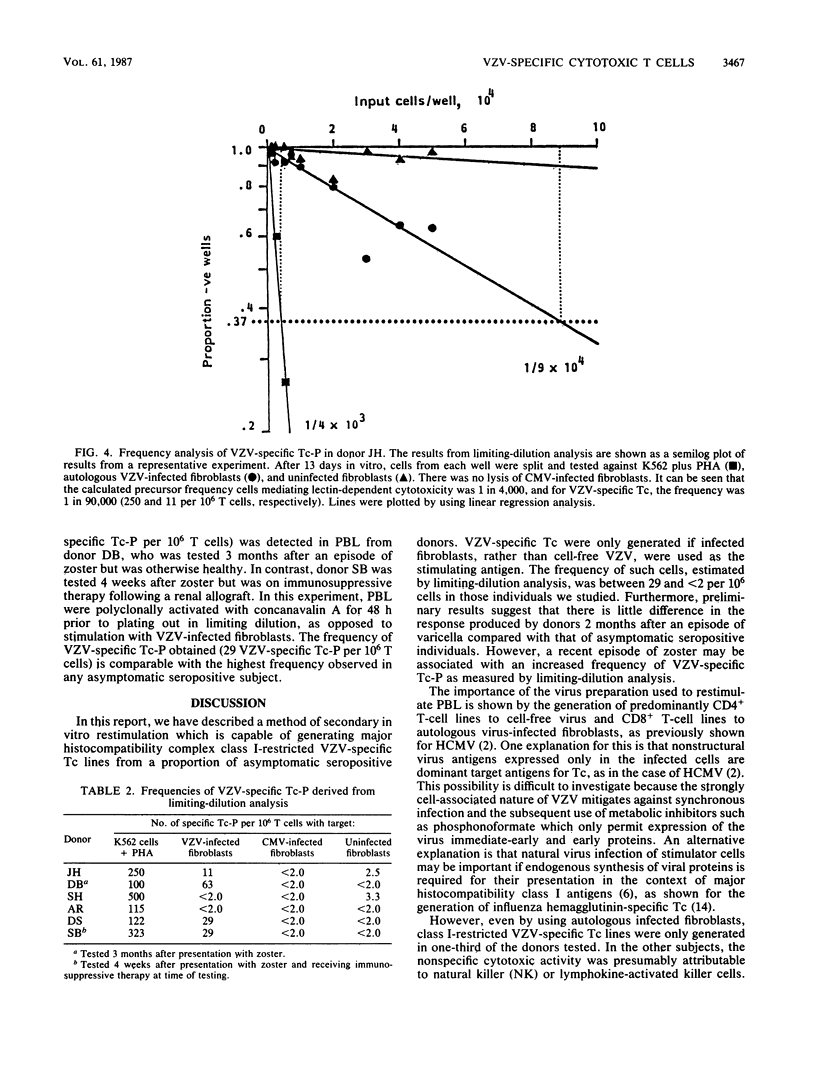
Abstract
The cytotoxic T-cell (Tc) response to varicella-zoster virus (VZV) is incompletely characterized. We investigated whether VZV-specific Tc restricted by class I products of the major histocompatibility complex can be generated from the peripheral blood of VZV-immune donors. Cell lines were established from peripheral blood lymphocytes (PBL) of seropositive donors by secondary in vitro restimulation. If cell-free VZV was used as the stimulating antigen, the resulting lines were predominantly CD4+ and did not show class I-restricted cytotoxicity; when autologous infected fibroblasts were used for in vitro stimulation, the resultant lines were usually cytotoxic, although in only 4 of 11 subjects tested was this cytotoxicity HLA restricted and virus specific. PBL were also tested for Tc activity without prior restimulation; VZV-specific Tc activity was only demonstrable in the PBL of a subject convalescent following zoster but not from subjects with recent varicella infection or from normal subjects. VZV-specific Tc precursor frequencies were then determined in six selected subjects by limiting-dilution analysis. A measurable frequency was detectable in four of the six seropositive subjects, ranging from 11/10(6) T cells in an asymptomatic carrier, to 63/10(6) T cells in a subject with recent zoster. We conclude that virus-specific major histocompatibility complex class I-restricted Tc precursors may be present in the peripheral blood of normal individuals seropositive for VZV but at a frequency lower than that for other herpesviruses with nonneuronal sites of latency.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvin A. M., Koropchak C. M., Williams B. R., Grumet F. C., Foung S. K. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986 Sep;154(3):422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Morris S., Page J. D., Sissons J. G. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur J Immunol. 1983 Oct;13(10):804–809. doi: 10.1002/eji.1830131005. [DOI] [PubMed] [Google Scholar]
- Bowden R. A., Levin M. J., Giller R. H., Tubergen D. G., Hayward A. R. Lysis of varicella zoster virus infected cells by lymphocytes from normal humans and immunosuppressed pediatric leukaemic patients. Clin Exp Immunol. 1985 May;60(2):387–395. [PMC free article] [PubMed] [Google Scholar]
- Falkoff R. M., Peters M., Fauci A. S. T cell enrichment and depletion of human peripheral blood mononuclear cell preparations. Unexpected findings in the study of the functional activities of the separated populations. J Immunol Methods. 1982;50(1):39–49. doi: 10.1016/0022-1759(82)90302-7. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Vafai A., Shtram Y., Becker Y., Devlin M., Wellish M. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983 Dec 1;306(5942):478–480. doi: 10.1038/306478a0. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Herberger M., Lazslo M. Cellular interactions in the lysis of varicella-zoster virus infected human fibroblasts. Clin Exp Immunol. 1986 Jan;63(1):141–146. [PMC free article] [PubMed] [Google Scholar]
- Hayward A. R., Pontesilli O., Herberger M., Laszlo M., Levin M. Specific lysis of varicella zoster virus-infected B lymphoblasts by human T cells. J Virol. 1986 Apr;58(1):179–184. doi: 10.1128/jvi.58.1.179-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai T., Chiba Y., Wataya Y., Hanazono H., Chiba S., Nakao T. Development and characteristics of the cellular immune response to infection with varicella-zoster virus. J Infect Dis. 1980 Jan;141(1):7–13. doi: 10.1093/infdis/141.1.7. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Stevens D. A. Viral infections in man associated with acquired immunological deficiency states. Fed Proc. 1971 Nov-Dec;30(6):1858–1864. [PubMed] [Google Scholar]
- Moretta A., Pantaleo G., Moretta L., Mingari M. C., Cerottini J. C. Quantitative assessment of the pool size and subset distribution of cytolytic T lymphocytes within human resting or alloactivated peripheral blood T cell populations. J Exp Med. 1983 Aug 1;158(2):571–585. doi: 10.1084/jem.158.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. A., Yoonessi S., O'Malley J., Freeman A., Gershon A., Ogra P. L. Cell-mediated immunity to varicella-zoster virus infection in subjects with lymphoma or leukemia. J Pediatr. 1979 Feb;94(2):223–230. doi: 10.1016/s0022-3476(79)80828-8. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Esber E., Saral R., Manischewitz J. F., Rogers J. L., Rook A. H., Santos G. W., Burns W. H. HLA-restricted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981 May;126(5):2036–2041. [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Wallace L. E., Rowe M., Misko I. S., Epstein M. A., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4216–4221. [PubMed] [Google Scholar]
- Rickinson A. B., Wallace L. E., Epstein M. A. HLA-restricted T-cell recognition of Epstein-Barr virus-infected B cells. Nature. 1980 Feb 28;283(5750):865–867. doi: 10.1038/283865a0. [DOI] [PubMed] [Google Scholar]
- Sørensen O. S., Haahr S., Møller-Larsen A., Wildenhoff K. Cell-mediated and humoral immunity to herpesviruses during and after herpes zoster infections. Infect Immun. 1980 Aug;29(2):369–375. doi: 10.1128/iai.29.2.369-375.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q. Y., Rickinson A. B., Gaston J. S., Epstein M. A. In vitro analysis of the Epstein-Barr virus: host balance in long-term renal allograft recipients. Int J Cancer. 1985 Jan 15;35(1):43–49. doi: 10.1002/ijc.2910350108. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Shiroguchi T., Kobayashi Y. HLA-restricted T lymphocyte-mediated cytotoxicity against herpes simplex virus-infected cells in humans. Infect Immun. 1983 Apr;40(1):190–197. doi: 10.1128/iai.40.1.190-197.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Lasky L. A., Moss B. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J Virol. 1986 Aug;59(2):506–509. doi: 10.1128/jvi.59.2.506-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


