Abstract
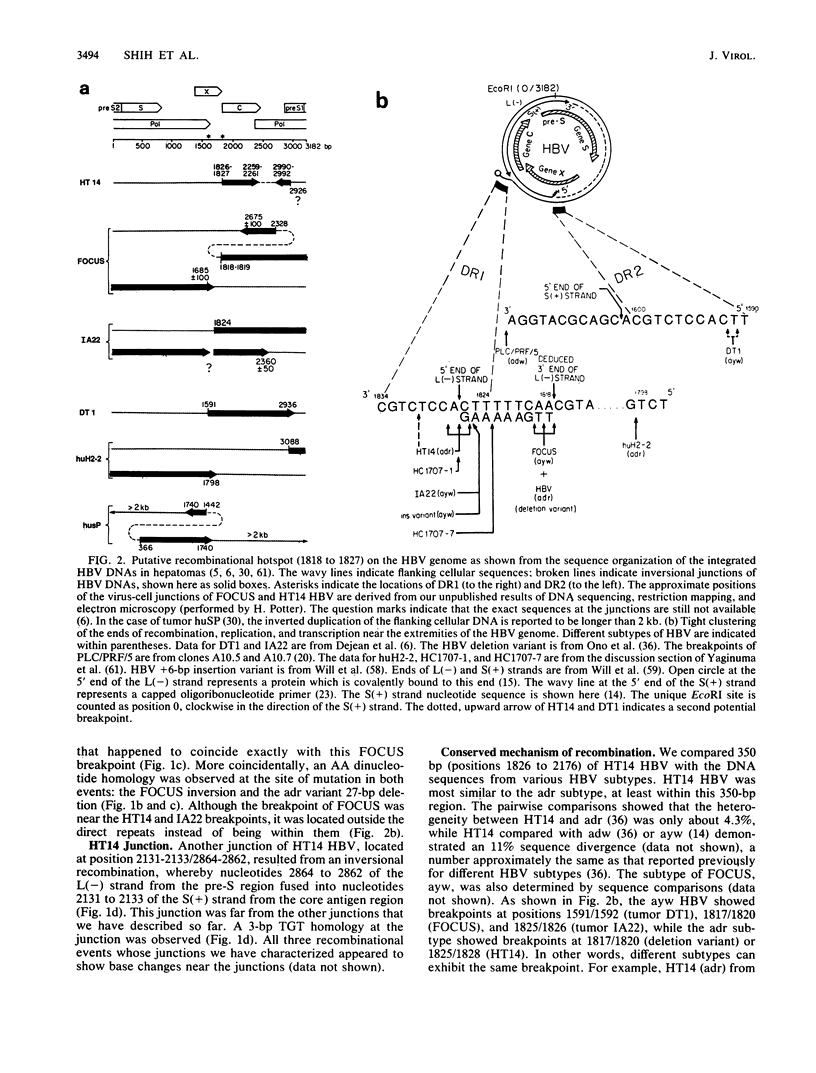
The open circular genome of human hepatitis B virus (HBV) is known to contain a partially double-stranded DNA with a single-stranded gap region of variable length. This circular structure of the genome is maintained by base-pairing of the 5' ends of the two DNA stands, the long or L(-) strand and the short or S(+) strand. By cloning, mapping, and sequencing studies, we have localized three recombinational junctions of the integrated HBV in two hepatoma samples, HT14 and FOCUS. Breakpoints of recombination derived from these results and those of others appear to be clustered and coincidental with the identified 5' or the deduced 3' end of the long-strand DNA, respectively. Statistical analysis of these results supports the hypothesis that integration preferentially occurs in an extremely narrow region on the HBV genome. This site-specific recombinational mechanism appears to be conserved among different HBV subtypes. No extensive sequence homology was found between each pair of the recombining parental molecules; however, at the site of crossover, 2- to 3-base-pair junctional homology was consistently observed. Examination of the patterns of the integrated HBV DNAs allowed us to categorize these various patterns into four different groups according to their end specificity and strand polarity. The molecular form of relaxed circle is proposed to be one major substrate for HBV integration. The effect of free strand in the integration of HBV is emphasized in this model. Unlike any other known DNA animal viruses, the site specificity of HBV integration appears to be similar to that of the retroviruses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Cummings I. W., Browne J. K., Salser W. A., Tyler G. V., Snyder R. L., Smolec J. M., Summers J. Isolation, characterization, and comparison of recombinant DNAs derived from genomes of human hepatitis B virus and woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1842–1846. doi: 10.1073/pnas.77.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Dejean A., Sonigo P., Wain-Hobson S., Tiollais P. Specific hepatitis B virus integration in hepatocellular carcinoma DNA through a viral 11-base-pair direct repeat. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5350–5354. doi: 10.1073/pnas.81.17.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Gough N. M., Cameron C. H., Murray K. Structure of the hepatitis B virus genome. J Virol. 1983 Aug;47(2):337–343. doi: 10.1128/jvi.47.2.337-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. Mapping the major transcripts of ground squirrel hepatitis virus: the presumptive template for reverse transcriptase is terminally redundant. Cell. 1985 Aug;42(1):297–308. doi: 10.1016/s0092-8674(85)80125-2. [DOI] [PubMed] [Google Scholar]
- Feitelson M. A., Millman I., Halbherr T., Simmons H., Blumberg B. S. A newly identified hepatitis B type virus in tree squirrels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2233–2237. doi: 10.1073/pnas.83.7.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- He L., Isselbacher K. J., Wands J. R., Goodman H. M., Shih C., Quaroni A. Establishment and characterization of a new human hepatocellular carcinoma cell line. In Vitro. 1984 Jun;20(6):493–504. doi: 10.1007/BF02619623. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Koike K. Complete nucleotide sequence of hepatitis B virus DNA of subtype adr and its conserved gene organization. Gene. 1984 Oct;30(1-3):227–232. doi: 10.1016/0378-1119(84)90124-0. [DOI] [PubMed] [Google Scholar]
- Koch S., von Loringhoven A. F., Hofschneider P. H., Koshy R. Amplification and rearrangement in hepatoma cell DNA associated with integrated hepatitis B virus DNA. EMBO J. 1984 Sep;3(9):2185–2189. doi: 10.1002/j.1460-2075.1984.tb02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy R., Koch S., von Loringhoven A. F., Kahmann R., Murray K., Hofschneider P. H. Integration of hepatitis B virus DNA: evidence for integration in the single-stranded gap. Cell. 1983 Aug;34(1):215–223. doi: 10.1016/0092-8674(83)90152-6. [DOI] [PubMed] [Google Scholar]
- Landers T. A., Greenberg H. B., Robinson W. S. Structure of hepatitis B Dane particle DNA and nature of the endogenous DNA polymerase reaction. J Virol. 1977 Aug;23(2):368–376. doi: 10.1128/jvi.23.2.368-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Van Davelaar M. J., Knight S. S., Salazar F. H., Garcia G., Popper H., Robinson W. S. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4543–4546. doi: 10.1073/pnas.83.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Aldrich C., Summers J., Taylor J. M. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3997–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Common evolutionary origin of hepatitis B virus and retroviruses. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2531–2535. doi: 10.1073/pnas.83.8.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Hepatitis B virus DNA forms in nuclear and cytoplasmic fractions of infected human liver. Virology. 1984 Sep;137(2):390–399. doi: 10.1016/0042-6822(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Mizusawa H., Taira M., Yaginuma K., Kobayashi M., Yoshida E., Koike K. Inversely repeating integrated hepatitis B virus DNA and cellular flanking sequences in the human hepatoma-derived cell line huSP. Proc Natl Acad Sci U S A. 1985 Jan;82(1):208–212. doi: 10.1073/pnas.82.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Kimber K. L., Summers J. W., Mason W. S. Mapping of the cohesive overlap of duck hepatitis B virus DNA and of the site of initiation of reverse transcription. J Virol. 1984 Jul;51(1):181–191. doi: 10.1128/jvi.51.1.181-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möröy T., Etiemble J., Trépo C., Tiollais P., Buendia M. A. Transcription of woodchuck hepatitis virus in the chronically infected liver. EMBO J. 1985 Jun;4(6):1507–1514. doi: 10.1002/j.1460-2075.1985.tb03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Ogston C. W., Jonak G. J., Rogler C. E., Astrin S. M., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from hepatocellular carcinomas of woodchucks. Cell. 1982 Jun;29(2):385–394. doi: 10.1016/0092-8674(82)90155-6. [DOI] [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby L. R., Hung P. P., Mao J. C., Ling C. M. Rolling circular DNA associated with Dane particles in hepatitis B virus. Nature. 1975 May 1;255(5503):84–85. doi: 10.1038/255084a0. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T. Retroviral DNA integration. Cell. 1985 Aug;42(1):5–6. doi: 10.1016/s0092-8674(85)80092-1. [DOI] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Gagnon G. C. Patterns of proviral insertion and deletion in avian leukosis virus-induced lymphomas. J Virol. 1986 Jan;57(1):28–36. doi: 10.1128/jvi.57.1.28-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Clayton D. A., Greenman R. L. DNA of a human hepatitis B virus candidate. J Virol. 1974 Aug;14(2):384–391. doi: 10.1128/jvi.14.2.384-391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler C. E., Sherman M., Su C. Y., Shafritz D. A., Summers J., Shows T. B., Henderson A., Kew M. Deletion in chromosome 11p associated with a hepatitis B integration site in hepatocellular carcinoma. Science. 1985 Oct 18;230(4723):319–322. doi: 10.1126/science.2996131. [DOI] [PubMed] [Google Scholar]
- Rogler C. E., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from a chronically infected liver. J Virol. 1984 Jun;50(3):832–837. doi: 10.1128/jvi.50.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler F., Robinson W. S. Hepatitis B viral DNA molecules have cohesive ends. J Virol. 1979 Oct;32(1):226–233. doi: 10.1128/jvi.32.1.226-233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981 Mar-Apr;1(2):179–183. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Tuttleman J. S., Pourcel C., Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986 Nov 7;47(3):451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Will H., Kuhn C., Cattaneo R., Schaller H. Structure and function of the hepatitis B virus genome. Princess Takamatsu Symp. 1982;12:237–247. [PubMed] [Google Scholar]
- Will H., Reiser W., Weimer T., Pfaff E., Büscher M., Sprengel R., Cattaneo R., Schaller H. Replication strategy of human hepatitis B virus. J Virol. 1987 Mar;61(3):904–911. doi: 10.1128/jvi.61.3.904-911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J., Darling S. M., Erickson R. P., Craig I. W., Buckle V. J., Rigby P. W., Willard H. F., Goodfellow P. N. Isolation and characterization of an alphoid centromeric repeat family from the human Y chromosome. J Mol Biol. 1985 Apr 20;182(4):477–485. doi: 10.1016/0022-2836(85)90234-7. [DOI] [PubMed] [Google Scholar]
- Yaginuma K., Kobayashi M., Yoshida E., Koike K. Hepatitis B virus integration in hepatocellular carcinoma DNA: duplication of cellular flanking sequences at the integration site. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4458–4462. doi: 10.1073/pnas.82.13.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka O., Omata M., Zhou Y. Z., Imazeki F., Okuda K. Duck hepatitis B virus DNA in liver and serum of Chinese ducks: integration of viral DNA in a hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5180–5184. doi: 10.1073/pnas.82.15.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemer M., Garcia P., Shaul Y., Rutter W. J. Sequence of hepatitis B virus DNA incorporated into the genome of a human hepatoma cell line. J Virol. 1985 Mar;53(3):885–892. doi: 10.1128/jvi.53.3.885-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


