Abstract
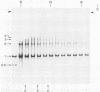
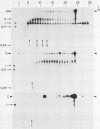
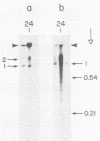
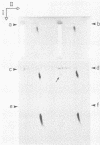
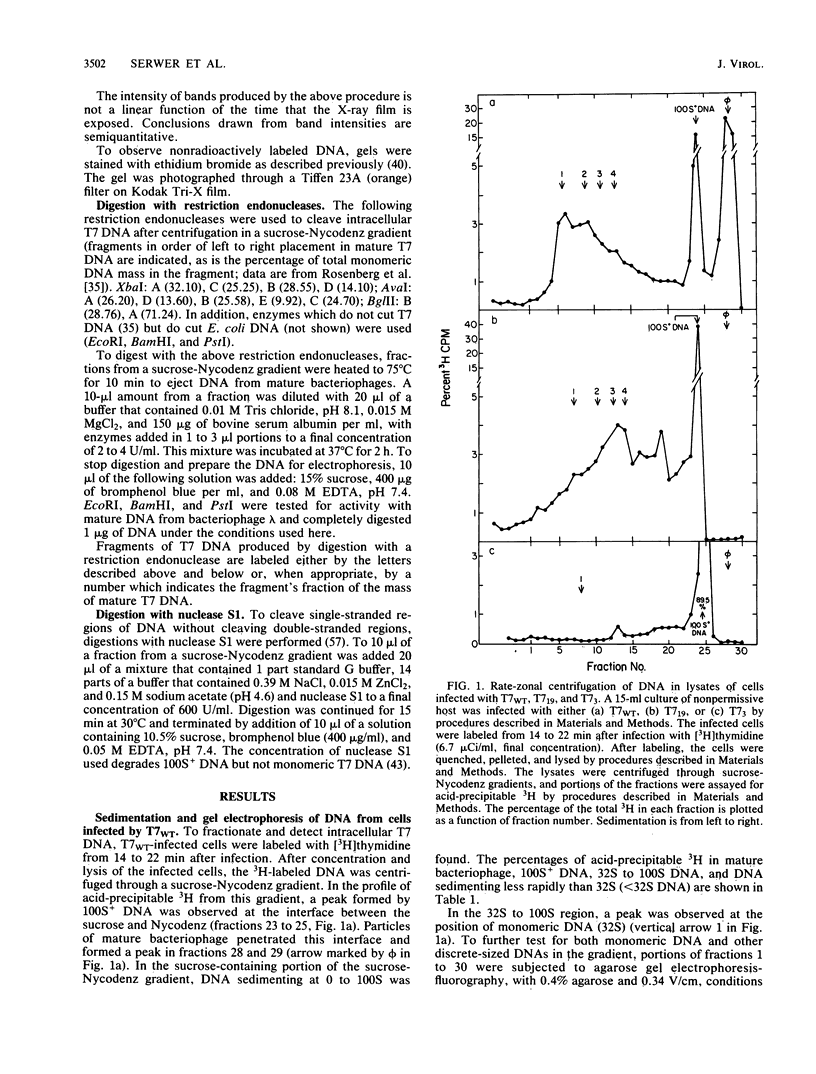
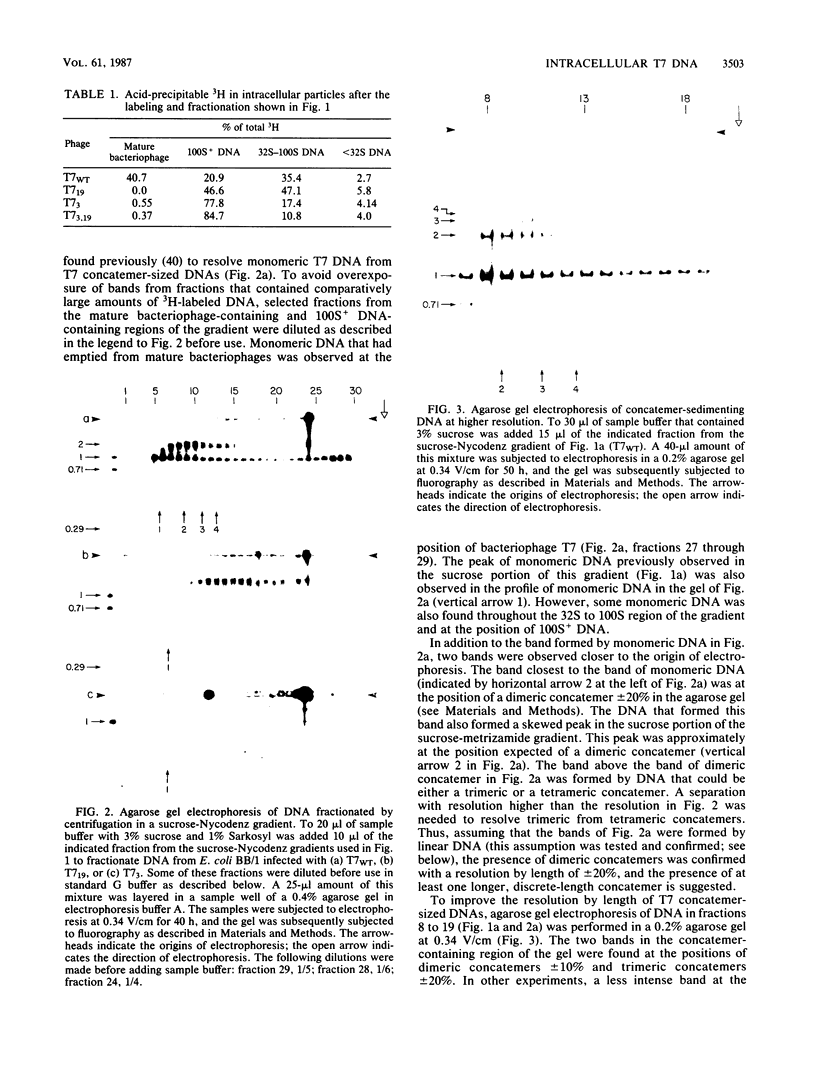
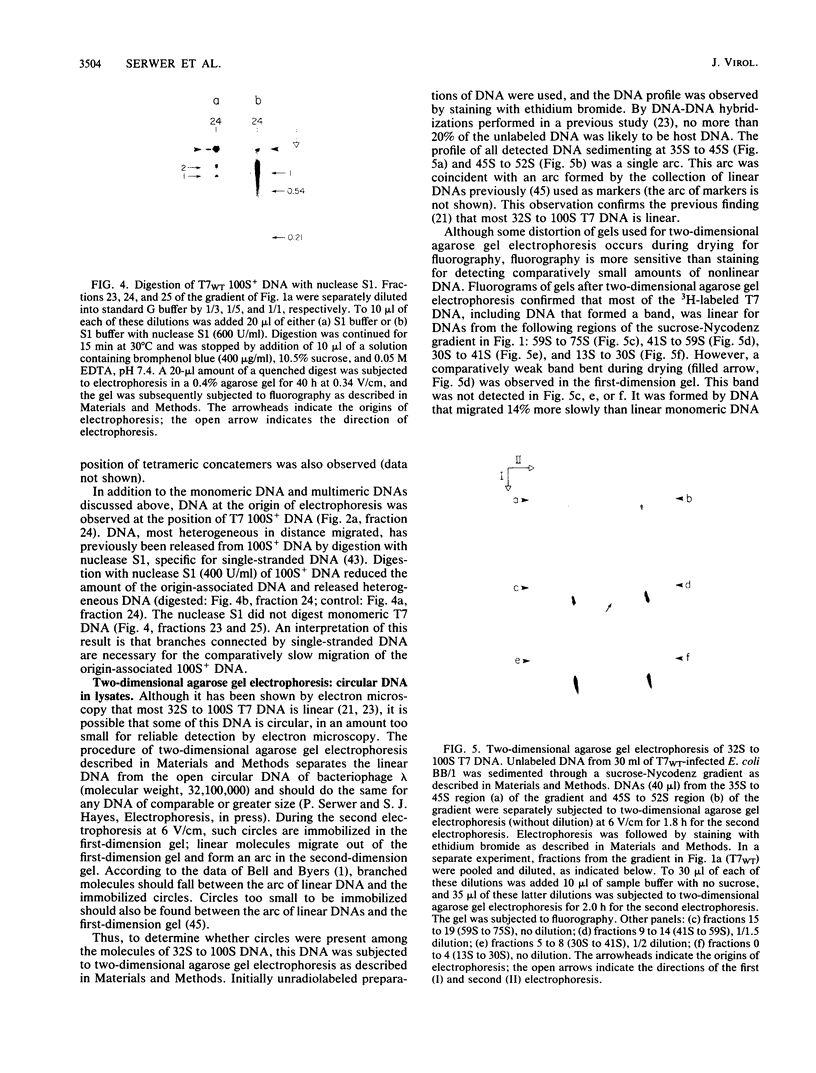
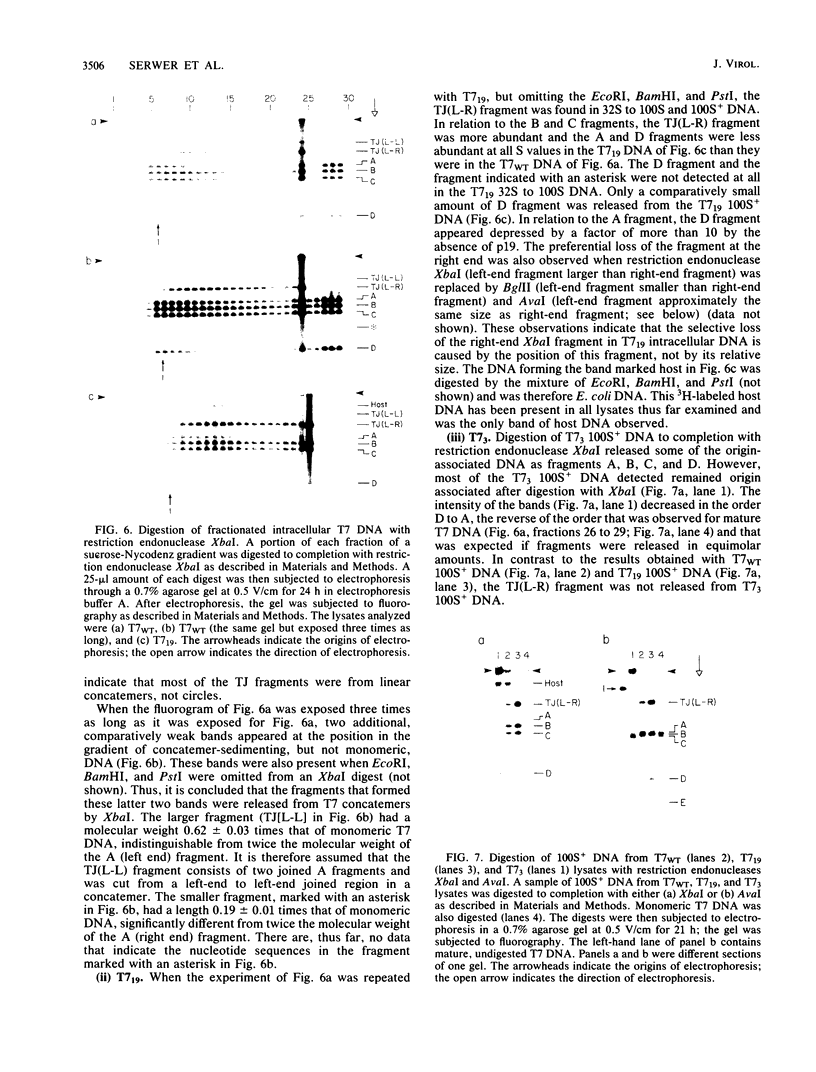
By use of rate-zonal centrifugation, followed by either one- or two-dimensional agarose gel electrophoresis, the forms of intracellular bacteriophage T7 DNA produced by replication, recombination, and packaging have been analyzed. Previous studies had shown that at least some intracellular DNA with sedimentation coefficients between 32S (the S value of mature T7 DNA) and 100S is concatemeric, i.e., linear and longer than mature T7 DNA. The analysis presented here confirmed that most of this DNA is linear, but also revealed a significant amount of circular DNA. The data suggest that these circles are produced during DNA packaging. It is proposed that circles are produced after a capsid has bound two sequential genomes in a concatemer. The size distribution of the linear, concatemeric DNA had peaks at the positions of dimeric and trimeric concatemers. Restriction endonuclease analysis revealed that most of the mature T7 DNA subunits of concatemers were joined left end to right end. However, these data also suggest that a comparatively small amount of left-end to left-end joining occurs, possibly by blunt-end ligation. A replicating form of T7 DNA that had an S value greater than 100 (100S+ DNA) was also found to contain concatemers. However, some of the 100S+ DNA, probably the most branched component, remained associated with the origin after agarose gel electrophoresis. It has been found that T7 protein 19, known to be required for DNA packaging, was also required to prevent loss, probably by nucleolytic degradation, of the right end of all forms of intracellular T7 DNA. T7 gene 3 endonuclease, whose activity is required for both recombination of T7 DNA and degradation of host DNA, was required for the formation of the 32S to 100S molecules that behaved as concatemers during gel electrophoresis. In the absence of gene 3 endonuclease, the primary accumulation product was origin-associated 100S+ DNA with properties that suggest the accumulation of branches, primarily at the left end of mature DNA subunits within the 100S+ DNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell L., Byers B. Separation of branched from linear DNA by two-dimensional gel electrophoresis. Anal Biochem. 1983 Apr 15;130(2):527–535. doi: 10.1016/0003-2697(83)90628-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Center M. S., Studier F. W., Richardson C. C. The structural gene for a T7 endonuclease essential for phage DNA synthesis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):242–248. doi: 10.1073/pnas.65.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Davison P. F. THE EFFECT OF HYDRODYNAMIC SHEAR ON THE DEOXYRIBONUCLEIC ACID FROM T(2) AND T(4) BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1560–1568. doi: 10.1073/pnas.45.11.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D., Potter H. Molecular mechanisms in genetic recombination. Annu Rev Biochem. 1982;51:727–761. doi: 10.1146/annurev.bi.51.070182.003455. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Fisher H. W., Williams R. C. Electron microscopic visualization of nucleic acids and of their complexes with proteins. Annu Rev Biochem. 1979;48:649–679. doi: 10.1146/annurev.bi.48.070179.003245. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Fröhlich B., Powling A., Knippers R. Formation of concatemeric DNA in bacteriophage T7-infected bacteria. Virology. 1975 Jun;65(2):455–468. doi: 10.1016/0042-6822(75)90051-3. [DOI] [PubMed] [Google Scholar]
- Fuller C. W., Richardson C. C. Initiation of DNA replication at the primary origin of bacteriophage T7 by purified proteins. Site and direction of initial DNA synthesis. J Biol Chem. 1985 Mar 10;260(5):3185–3196. [PubMed] [Google Scholar]
- Hamada K., Fujisawa H., Minagawa T. A defined in vitro system for packaging of bacteriophage T3 DNA. Virology. 1986 May;151(1):119–123. doi: 10.1016/0042-6822(86)90109-1. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J. Electron microscopical studies of phage multiplication. II. Production of phage-related structures during multiplication of phages T2 and T4. Virology. 1957 Apr;3(2):256–274. doi: 10.1016/0042-6822(57)90092-2. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. The involvement of genes 3,4,5 and 6 in genetic recombination in bacteriophage T7. Virology. 1975 May;65(1):281–285. doi: 10.1016/0042-6822(75)90031-8. [DOI] [PubMed] [Google Scholar]
- Langman L., Paetkau V. Purification and structures of recombining and replicating bacteriophage T7 DNA. J Virol. 1978 Feb;25(2):562–569. doi: 10.1128/jvi.25.2.562-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman L., Paetkau V., Scraba D., Miller R. C., Jr, Roeder G. S., Sadowski P. D. The structure and maturation of intermediates in bacteriophage T7 DNA replication. Can J Biochem. 1978 Jun;56(6):508–516. doi: 10.1139/o78-078. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee D., Sadowski P. D. Genetic recombination of bacteriophage T7 in vivo studied by use of a simple physical assay. J Virol. 1981 Dec;40(3):839–847. doi: 10.1128/jvi.40.3.839-847.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Jr, Lee M. The role of bacteriophage T7 exonuclease (gene 6) in genetic recombination and production of concatemers. J Mol Biol. 1976 Feb 25;101(2):223–234. doi: 10.1016/0022-2836(76)90374-0. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Kemper B., Hays J., Weisberg R. A. T4 endonuclease VII cleaves holliday structures. Cell. 1982 Jun;29(2):357–365. doi: 10.1016/0092-8674(82)90152-0. [DOI] [PubMed] [Google Scholar]
- Paetkau V., Langman L., Bradley R., Scraba D., Miller R. C., Jr Folded, concatenated genomes as replication intermediates of bacteriophage T7 DNA. J Virol. 1977 Apr;22(1):130–141. doi: 10.1128/jvi.22.1.130-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powling A., Knippers R. Some functions involved in bacteriophage T7 genetic recombination. Mol Gen Genet. 1974;134(2):173–180. doi: 10.1007/BF00268418. [DOI] [PubMed] [Google Scholar]
- Ritchie D. A., Thomas C. A., Jr, MacHattie L. A., Wensink P. C. Terminal repetition in non-permuted T3 and T7 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):365–376. doi: 10.1016/s0022-2836(67)80111-6. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Simon M. N., Studier F. W., Roberts R. J. Survey and mapping of restriction endonuclease cleavage sites in bacteriophage T7 DNA. J Mol Biol. 1979 Dec 25;135(4):907–915. doi: 10.1016/0022-2836(79)90519-9. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Kerr C. Degradation of Escherichia coli B deoxyribonucleic acid after infection with deoxyribonucleic acid-defective amber mutants of bacteriophage T7. J Virol. 1970 Aug;6(2):149–155. doi: 10.1128/jvi.6.2.149-155.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Serwer P. Complexes between bacteriophage T7 capsids and T7 DNA. Virology. 1974 May;59(1):89–107. doi: 10.1016/0042-6822(74)90208-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. Electrophoresis of duplex deoxyribonucleic acid in multiple-concentration agarose gels: fractionation of molecules with molecular weights between 2 X 10(6) and 110 X 10(6). Biochemistry. 1980 Jun 24;19(13):3001–3004. doi: 10.1021/bi00554a026. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fast sedimenting bacteriophage T7 DNA from T7-infected Escherichia coli. Virology. 1974 May;59(1):70–88. doi: 10.1016/0042-6822(74)90207-4. [DOI] [PubMed] [Google Scholar]
- Serwer P., Greenhaw G. A., Allen J. L. Concatemers in a rapidly sedimenting, replicating bacteriophage T7 DNA. Virology. 1982 Dec;123(2):474–479. doi: 10.1016/0042-6822(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Serwer P. Two-dimensional agarose gel electrophoresis without gel manipulation. Anal Biochem. 1985 Jan;144(1):172–178. doi: 10.1016/0003-2697(85)90100-9. [DOI] [PubMed] [Google Scholar]
- Serwer P. Use of gel electrophoresis to characterize multimolecular aggregates. Methods Enzymol. 1986;130:116–132. doi: 10.1016/0076-6879(86)30010-7. [DOI] [PubMed] [Google Scholar]
- Serwer P., Watson R. H., Hayes S. J., Allen J. L. Comparison of the physical properties and assembly pathways of the related bacteriophages T7, T3 and phi II. J Mol Biol. 1983 Oct 25;170(2):447–469. doi: 10.1016/s0022-2836(83)80157-0. [DOI] [PubMed] [Google Scholar]
- Sgaramella V., Ehrlich S. D. Use of the T4 polynucleotide ligase in the joining of flush-ended DNA segments generated by restriction endonucleases. Eur J Biochem. 1978 May 16;86(2):531–537. doi: 10.1111/j.1432-1033.1978.tb12336.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Measurement of DNA length by gel electrophoresis. Anal Biochem. 1979 Dec;100(2):319–323. doi: 10.1016/0003-2697(79)90235-5. [DOI] [PubMed] [Google Scholar]
- Stone J. C., Miller R. C., Jr Plasmid-phage recombination in T7 infected Escherichia coli. Virology. 1984 Sep;137(2):305–313. doi: 10.1016/0042-6822(84)90222-8. [DOI] [PubMed] [Google Scholar]
- Strätling W., Krause E., Knippers R. Fast sedimenting deoxyribonucleic acid in bacteriophage T7-infected cells. Virology. 1973 Jan;51(1):109–119. doi: 10.1016/0042-6822(73)90371-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Dunn J. J. Organization and expression of bacteriophage T7 DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):999–1007. doi: 10.1101/sqb.1983.047.01.114. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr, Kelly T. J., Jr, Rhoades M. The intracellular forms of T7 and P22 DNA molecules. Cold Spring Harb Symp Quant Biol. 1968;33:417–424. doi: 10.1101/sqb.1968.033.01.048. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ogawa H. Intermediates in genetic recombination of bacteriophage T7 DNA. J Mol Biol. 1977 Jan 25;109(3):423–426. doi: 10.1016/s0022-2836(77)80021-1. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wever G. H., Fischer H., Hinkle D. C. Bacteriophage T7 DNA replication in vitro. Electron micrographic analysis of T7 DNA synthesized with purified proteins. J Biol Chem. 1980 Aug 25;255(16):7965–7972. [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M., Fujisawa H., Minagawa T. Isolation and characterization of bacteriophage T3/T7 hybrids and their use in studies on molecular basis of DNA-packaging specificity. Virology. 1985 Jul 30;144(2):502–515. doi: 10.1016/0042-6822(85)90290-9. [DOI] [PubMed] [Google Scholar]
- de Massy B., Studier F. W., Dorgai L., Appelbaum E., Weisberg R. A. Enzymes and sites of genetic recombination: studies with gene-3 endonuclease of phage T7 and with site-affinity mutants of phage lambda. Cold Spring Harb Symp Quant Biol. 1984;49:715–726. doi: 10.1101/sqb.1984.049.01.081. [DOI] [PubMed] [Google Scholar]