Abstract
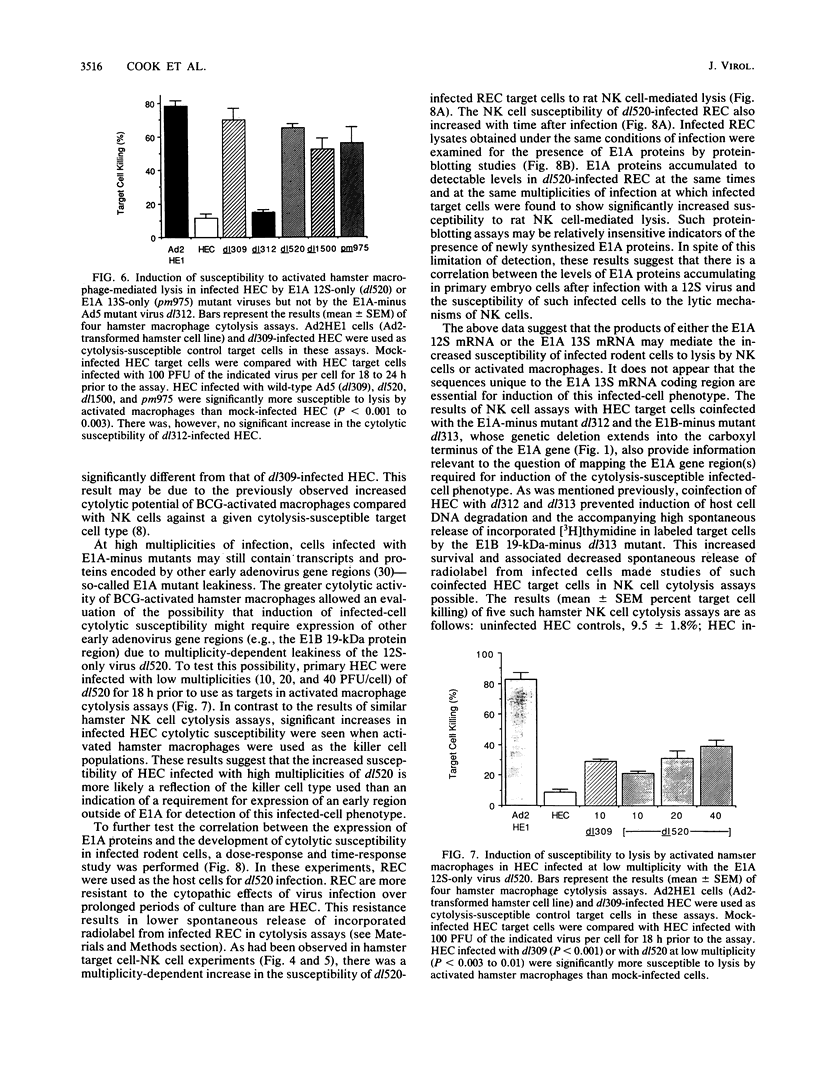
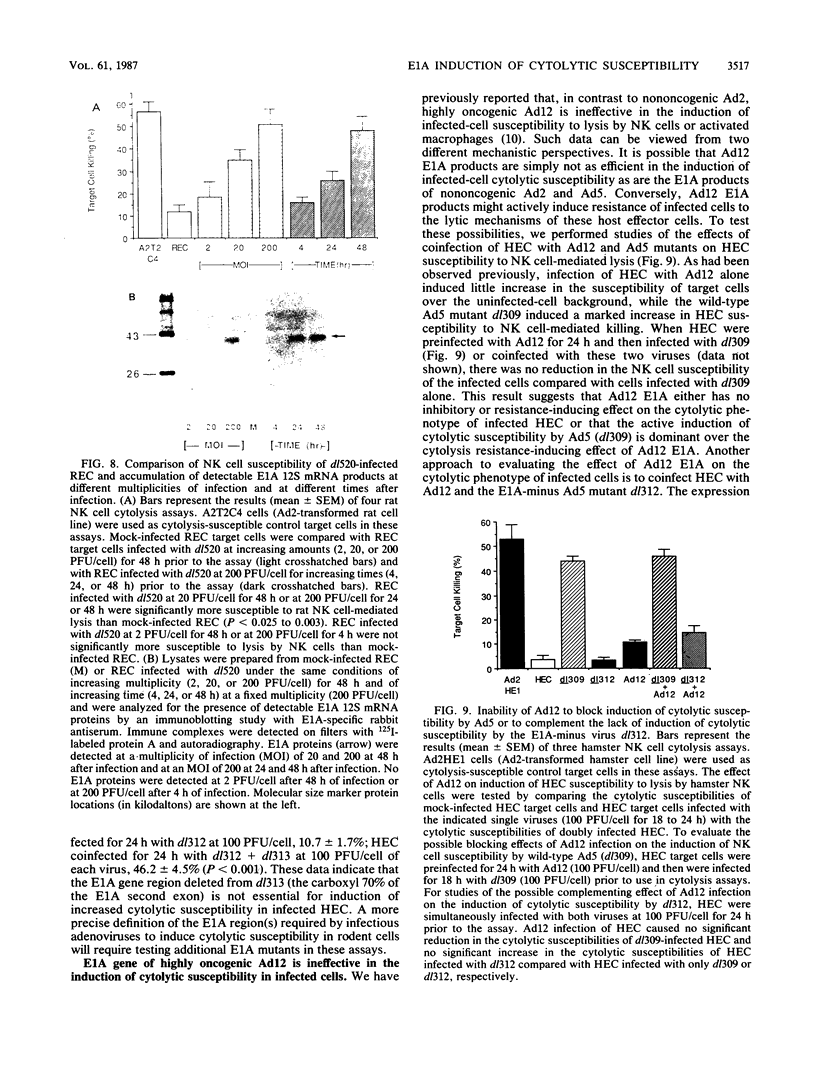
Rodent cells immortalized by the E1A gene of nononcogenic adenoviruses are susceptible to lysis by natural killer (NK) cells and activated macrophages. This cytolysis-susceptible phenotype may contribute to the rejection of adenovirus-transformed cells by immunocompetent animals. Such increased cytolytic susceptibility has also been observed with infected rodent cells. This infection model provided a means to study the role of E1A gene products in induction of cytolytic susceptibility without cell selection during transformation. Deletion mutations outside of the E1A gene had no effect on adenovirus type 2 (Ad2) or Ad5 induction of cytolytic susceptibility in infected hamster cells, while E1A-minus mutant viruses could not induce this phenotype. E1A mutant viruses that induced expression of either E1A 12S or 13S mRNA in infected cells were competent to induce cytolytic susceptibility. Furthermore, there was a correlation between the accumulation of E1A gene products in Ad5-infected cells and the level of susceptibility of such target cells to lysis by NK cells. The results of coinfection studies indicated that the E1A gene products of highly oncogenic Ad12 could not complement the lack of induction of cytolytic susceptibility by E1A-minus Ad5 virus in infected cells and also could not block induction of this infected-cell phenotype by Ad5. These data suggest that expression of the E1A gene of nononcogenic adenoviruses may cause the elimination of infected cells by the immunologically nonspecific host inflammatory cell response prior to cellular transformation. The lack of induction of this cytolysis-susceptible phenotype by Ad12 E1A may result in an increased persistence of Ad12-infected cells in vivo and may lead to an increased Ad12-transformed cell burden for the host.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards R., Houweling A., Schrier P. I., Bos J. L., Van der Eb A. J. Characterization of cells transformed by Ad5/Ad12 hybrid early region I plasmids. Virology. 1982 Jul 30;120(2):422–432. doi: 10.1016/0042-6822(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Rodgers B., Morris S., Graham S., Sissons J. G. Lysis of human cytomegalovirus infected fibroblasts by natural killer cells: demonstration of an interferon-independent component requiring expression of early viral proteins and characterization of effector cells. J Immunol. 1985 Apr;134(4):2695–2701. [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Challberg S. S., Ketner G. Deletion mutants of adenovirus 2: isolation and initial characterization of virus carrying mutations near the right end of the viral genome. Virology. 1981 Oct 15;114(1):196–209. doi: 10.1016/0042-6822(81)90265-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Flint S. J. Partition of E1A proteins between soluble and structural fractions of adenovirus-infected and -transformed cells. J Virol. 1986 Dec;60(3):1018–1026. doi: 10.1128/jvi.60.3.1018-1026.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. L., Patek P. Q., Cohn M. Tumorigenicity and lysis by natural killers. J Exp Med. 1981 Jan 1;153(1):89–106. doi: 10.1084/jem.153.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., Hibbs J. B., Jr, Lewis A. M., Jr DNA virus-transformed hamster cell--host effector cell interactions: level of resistance to cytolysis correlated with tumorigenicity. Int J Cancer. 1982 Dec 15;30(6):795–803. doi: 10.1002/ijc.2910300619. [DOI] [PubMed] [Google Scholar]
- Cook J. L., Lewis A. M., Jr Differential NK cell and macrophage killing of hamster cells infected with nononcogenic or oncogenic adenovirus. Science. 1984 May 11;224(4649):612–615. doi: 10.1126/science.6710160. [DOI] [PubMed] [Google Scholar]
- Cook J. L., Lewis A. M., Jr Host response to adenovirus 2-transformed hamster embryo cells. Cancer Res. 1979 May;39(5):1455–1461. [PubMed] [Google Scholar]
- Cook J. L., Walker T. A., Lewis A. M., Jr, Ruley H. E., Graham F. L., Pilder S. H. Expression of the adenovirus E1A oncogene during cell transformation is sufficient to induce susceptibility to lysis by host inflammatory cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6965–6969. doi: 10.1073/pnas.83.18.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Tsukamoto A., Montell C., Berk A. J. Enhanced expression of adenovirus transforming proteins. J Virol. 1982 Oct;44(1):276–285. doi: 10.1128/jvi.44.1.276-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K. P., Overhauser J., Babiss L. E., Ginsberg H. S., Jones N. C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Rosenberg M., Westphal H. Functions of purified E1A protein microinjected into mammalian cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6988–6992. doi: 10.1073/pnas.81.22.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Cook J. L. A new role for DNA virus early proteins in viral carcinogenesis. Science. 1985 Jan 4;227(4682):15–20. doi: 10.1126/science.3843807. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Cook J. L. The interface between adenovirus-transformed cells and cellular immune response in the challenged host. Curr Top Microbiol Immunol. 1984;110:1–22. doi: 10.1007/978-3-642-46494-2_1. [DOI] [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Morse S. S., McGeorge M. G. Macrophage extrinsic antiviral activity during herpes simplex virus infection. J Gen Virol. 1980 Feb;46(2):291–300. doi: 10.1099/0022-1317-46-2-291. [DOI] [PubMed] [Google Scholar]
- Persson H., Katze M. G., Philipson L. Purification of a native membrane-associated adenovirus tumor antigen. J Virol. 1982 Jun;42(3):905–917. doi: 10.1128/jvi.42.3.905-917.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Bloom B. R. Natural killer cells in resistance to virus-infected cells. Springer Semin Immunopathol. 1982;4(4):397–414. doi: 10.1007/BF02053741. [DOI] [PubMed] [Google Scholar]
- Raska K., Jr, Gallimore P. H. An inverse relation of the oncogenic potential of adenovirus-transformed cells and their sensitivity to killing by syngeneic natural killer cells. Virology. 1982 Nov;123(1):8–18. doi: 10.1016/0042-6822(82)90290-2. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Branton P. E., Graham F. L. The kinetics of synthesis of early viral proteins in KB cells infected with wild-type and transformation-defective host-range mutants of human adenovirus type 5. J Gen Virol. 1984 Mar;65(Pt 3):585–597. doi: 10.1099/0022-1317-65-3-585. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Complementation of adenovirus type 5 host range mutants by adenovirus type 12 in coinfected HeLa and BHK-21 cells. J Virol. 1981 Apr;38(1):191–197. doi: 10.1128/jvi.38.1.191-197.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Föhring B., Shenk T. E., Raska K., Jr Tumorigenicity of adenovirus-transformed cells: region E1A of adenovirus 12 confers resistance to natural killer cells. Virology. 1985 Dec;147(2):413–421. doi: 10.1016/0042-6822(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Schmitt R. C., Fahnestock M. L., Lewis J. B. Differential nuclear localization of the major adenovirus type 2 E1a proteins. J Virol. 1987 Feb;61(2):247–255. doi: 10.1128/jvi.61.2.247-255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Saito I., Maruyama K., Shimojo H. Isolation of a nondefective recombinant between adenovirus type 5 and early region 1A of adenovirus type 12. J Virol. 1983 May;46(2):632–637. doi: 10.1128/jvi.46.2.632-637.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Woodward J. G., Frelinger J. A. Macrophage antiviral activity: extrinsic versus intrinsic activity. Infect Immun. 1982 May;36(2):672–677. doi: 10.1128/iai.36.2.672-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Stillman B. Expression of adenovirus E1B mutant phenotypes is dependent on the host cell and on synthesis of E1A proteins. J Virol. 1987 Feb;61(2):426–435. doi: 10.1128/jvi.61.2.426-435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Shenk T. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 1984 Aug;3(8):1907–1912. doi: 10.1002/j.1460-2075.1984.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YABE Y., TRENTIN J. J., TAYLOR G. Cancer induction in hamsters by human type 12 adenovirus. Effect of age and of virus dose. Proc Soc Exp Biol Med. 1962 Nov;111:343–344. doi: 10.3181/00379727-111-27786. [DOI] [PubMed] [Google Scholar]