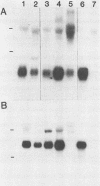
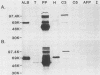
Abstract
Primary cultures of hepatocytes isolated by collagenase perfusion of adult rats were transformed by infection with adenovirus type 5 or transfection with adenovirus DNA. Total virion DNA or recombinant plasmid DNA containing the adenovirus E1A and E1B genes transformed hepatocytes at comparable frequencies. No foci of replicating hepatocytes were detected after transfection with a plasmid containing the E1A gene alone. The frequency of transformation by the adenovirus E1A and E1B genes was dependent on the composition of the culture medium. Transformation occurred at a low frequency when the transfected hepatocytes were maintained in a chemically defined medium (CDM), but the frequency was enhanced 8- to 10-fold when the cells were maintained in (i) serum-supplemented medium or (ii) CDM supplemented with epidermal growth factor. Cell lines derived from the adenovirus-transformed colonies of hepatocytes expressed adenovirus E1A and E1B RNAs. When hepatocytes were maintained in CDM supplemented with dimethyl sulfoxide and transfected with plasmids containing the E1A and E1B genes, it was possible to derive cell lines that retained the ability to express several liver-specific genes, including albumin, transferrin, hemopexin, and the third component of complement. The amount of albumin secreted per cell varied from 1 to 5 pg per cell per 24 h, and in one cell line it was below detectable levels by passage 9. Adenovirus-transformed hepatocytes were not tumorigenic when inoculated subcutaneously into neonatal syngeneic rats. We conclude that the adenovirus E1A and E1B genes are capable of transforming adult rat hepatocytes, a differentiated epithelial cell type.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUTWELL R. K. SOME BIOLOGICAL ASPECTS OF SKIN CARCINOGENISIS. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- Barker D. D., Berk A. J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987 Jan;156(1):107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Bernards R., Van der Eb A. J. Adenovirus: transformation and oncogenicity. Biochim Biophys Acta. 1984 Dec 14;783(3):187–204. doi: 10.1016/0167-4781(84)90029-0. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Byrd P., Brown K. W., Gallimore P. H. Malignant transformation of human embryo retinoblasts by cloned adenovirus 12 DNA. Nature. 1982 Jul 1;298(5869):69–71. doi: 10.1038/298069a0. [DOI] [PubMed] [Google Scholar]
- Chang S. E. In vitro transformation of human epithelial cells. Biochim Biophys Acta. 1986;823(3):161–194. doi: 10.1016/0304-419x(86)90001-6. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Keen J., Lane E. B., Taylor-Papadimitriou J. Establishment and characterization of SV40-transformed human breast epithelial cell lines. Cancer Res. 1982 May;42(5):2040–2053. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Feldhoff R. C., Taylor J. M., Jefferson L. S. Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. J Biol Chem. 1977 Jun 10;252(11):3611–3616. [PubMed] [Google Scholar]
- Fürstenberger G., Marks F. Growth stimulation and tumor promotion in skin. J Invest Dermatol. 1983 Jul;81(1 Suppl):157s–162s. doi: 10.1111/1523-1747.ep12540971. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Byrd P. J., Whittaker J. L., Grand R. J. Properties of rat cells transformed by DNA plasmids containing adenovirus type 12 E1 DNA or specific fragments of the E1 region: comparison of transforming frequencies. Cancer Res. 1985 Jun;45(6):2670–2680. [PubMed] [Google Scholar]
- Gallimore P. H., Grand R. J., Byrd P. J. Transformation of human embryo retinoblasts with simian virus 40, adenovirus and ras oncogenes. Anticancer Res. 1986 May-Jun;6(3 Pt B):499–508. [PubMed] [Google Scholar]
- Gallimore P. H. Interactions of adenovirus type 2 with rat embryo cells. Permissiveness, transformation and in vitro characteristics of adenovirus transformed rat embryo cells. J Gen Virol. 1974 Nov;25(2):263–273. doi: 10.1099/0022-1317-25-2-263. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., McDougall J. K., Chen L. B. In vitro traits of adenovirus-transformed cell lines and their relevance to tumorigenicity in nude mice. Cell. 1977 Apr;10(4):669–678. doi: 10.1016/0092-8674(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Georgoff I., Secott T., Isom H. C. Effect of simian virus 40 infection on albumin production by hepatocytes cultured in chemically defined medium and plated on collagen and non-collagen attachment surfaces. J Biol Chem. 1984 Aug 10;259(15):9595–9602. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Hennings H., Shores R., Wenk M. L., Spangler E. F., Tarone R., Yuspa S. H. Malignant conversion of mouse skin tumours is increased by tumour initiators and unaffected by tumour promoters. Nature. 1983 Jul 7;304(5921):67–69. doi: 10.1038/304067a0. [DOI] [PubMed] [Google Scholar]
- Hennings H., Yuspa S. H. Two-stage tumor promotion in mouse skin: an alternative interpretation. J Natl Cancer Inst. 1985 Apr;74(4):735–740. [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Isom H. C. DNA synthesis in isolated hepatocytes infected with herpesviruses. Virology. 1980 May;103(1):199–216. doi: 10.1016/0042-6822(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Isom H. C., Georgoff I. Quantitative assay for albumin-producing liver cells after simian virus 40 transformation of rat hepatocytes maintained in chemically defined medium. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6378–6382. doi: 10.1073/pnas.81.20.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom H. C., Liao W. S., Taylor J. M., Willwerth G. E., Eadline T. S. Rapid and selective shutoff of plasma protein production in herpes simplex virus type 2-infected hepatoma cells. Virology. 1983 Apr 30;126(2):548–562. doi: 10.1016/s0042-6822(83)80012-9. [DOI] [PubMed] [Google Scholar]
- Isom H. C., Secott T., Georgoff I., Woodworth C., Mummaw J. Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci U S A. 1985 May;82(10):3252–3256. doi: 10.1073/pnas.82.10.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Koi M., Barrett J. C. Loss of tumor-suppressive function during chemically induced neoplastic progression of Syrian hamster embryo cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5992–5996. doi: 10.1073/pnas.83.16.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Cooper G. M. Transforming activity of human tumor DNAs. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1181–1184. doi: 10.1073/pnas.78.2.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Brown K. W., Gallimore P. H. Adenovirus-cell interactions early after infection: in vitro characteristics and tumourigenicity of adenovirus type 2-transformed rat liver epithelial cells. J Gen Virol. 1982 Jan;58(Pt 1):73–81. doi: 10.1099/0022-1317-58-1-73. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Gallimore P. H. Tumorigenicity and in vitro characteristics of rat liver epithelial cells and their adenovirus-transformed derivatives. Int J Cancer. 1980 May 15;25(5):631–639. doi: 10.1002/ijc.2910250513. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Jay G., Arnstein P., Price F. M., Sanford K. K., Aaronson S. A. Neoplastic transformation of human epidermal keratinocytes by AD12-SV40 and Kirsten sarcoma viruses. Science. 1985 Mar 8;227(4691):1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Transformation of rodent cells by DNA extracted from transformation-defective adenovirus mutants. J Virol. 1983 Jun;46(3):1039–1044. doi: 10.1128/jvi.46.3.1039-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sammons D. W., Sanchez E., Darlington G. J. Immuno-overlay: a method for identification of hepatoma cell colonies that secrete albumin. In Vitro. 1980 Nov;16(11):918–924. doi: 10.1007/BF02619329. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Van Den Elsen P. J., Hertoghs J. J., Van Der Eb A. J. Characterization of tumor antigens in cells transformed by fragments of adenovirus type 5 DNA. Virology. 1979 Dec;99(2):372–385. doi: 10.1016/0042-6822(79)90016-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. J., Tevethia M. J. Identification of a human cytomegalovirus virus DNA segment that complements an adenovirus 5 immediate early mutant. Virology. 1986 Jun;151(2):329–338. doi: 10.1016/0042-6822(86)90053-x. [DOI] [PubMed] [Google Scholar]
- Spector D. J. Transcription of adenovirus 5 early region 1b is elevated in permissive cells infected by a mutant with an upstream deletion. J Virol. 1982 Nov;44(2):544–554. doi: 10.1128/jvi.44.2.544-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. W., Ziff E. B. Repression of insulin gene expression by adenovirus type 5 E1a proteins. Mol Cell Biol. 1987 Mar;7(3):1164–1170. doi: 10.1128/mcb.7.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen D. G., Gilmer T. M., Annab L. A., Barrett J. C. Evidence for multiple steps in neoplastic transformation of normal and preneoplastic Syrian hamster embryo cells following transfection with Harvey murine sarcoma virus oncogene (v-Ha-ras). Cancer Res. 1985 Feb;45(2):726–732. [PubMed] [Google Scholar]
- Woodworth C. D., Isom H. C. Regulation of albumin gene expression in a series of rat hepatocyte cell lines immortalized by simian virus 40 and maintained in chemically defined medium. Mol Cell Biol. 1987 Oct;7(10):3740–3748. doi: 10.1128/mcb.7.10.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C., Secott T., Isom H. C. Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res. 1986 Aug;46(8):4018–4026. [PubMed] [Google Scholar]
- Yoakum G. H., Lechner J. F., Gabrielson E. W., Korba B. E., Malan-Shibley L., Willey J. C., Valerio M. G., Shamsuddin A. M., Trump B. F., Harris C. C. Transformation of human bronchial epithelial cells transfected by Harvey ras oncogene. Science. 1985 Mar 8;227(4691):1174–1179. doi: 10.1126/science.3975607. [DOI] [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., de Pater S., Houweling A., van der Veer J., van der Eb A. The relationship between region E1a and E1b of human adenoviruses in cell transformation. Gene. 1982 May;18(2):175–185. doi: 10.1016/0378-1119(82)90115-9. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Bernards R. Transformation and oncogenicity by adenoviruses. Curr Top Microbiol Immunol. 1984;110:23–51. doi: 10.1007/978-3-642-46494-2_2. [DOI] [PubMed] [Google Scholar]