Abstract
We have shown previously that interleukin-4 (IL-4) protects TS1αβ cells from apoptosis, but very little is known about the mechanism by which IL-4 exerts this effect. We found that Akt activity, which is dependent on phosphatidylinositol 3 kinase, is reduced in IL-4-deprived TS1αβ cells. Overexpression of wild-type Akt or a constitutively active Akt mutant protects cells from IL-4 deprivation-induced apoptosis. Readdition of IL-4 before the commitment point is able to restore Akt activity. We also show expression and c-Jun N-terminal kinase 2 activation after IL-4 deprivation. Overexpression of the constitutively activated Akt mutant in IL-4-deprived cells correlates with inhibition of c-Jun N-terminal kinase 2 activity. Finally, TS1αβ survival is independent of Bcl-2, Bcl-x, or Bax.
INTRODUCTION
Interleukin-4 (IL-4) is a pleiotropic cytokine, produced predominantly by T cells, mast cells, and basophils, that stimulates proliferation of T cells, B cells, and mast cells (Seder et al., 1981; Howard et al., 1982; Brown et al., 1987; Paul, 1991). The biological functions of IL-4 are mediated via its binding to a specific cell surface receptor. This widely distributed receptor consists of two chains that are members of the type I cytokine receptor superfamily (Cosman, 1993), a ligand-binding chain (IL-4Rα), and the common γ chain, which is shared with the IL-2, IL-7, IL-9, and IL-15 receptors (Noguchi et al., 1993; Kondo et al., 1993, 1994; Russell et al., 1993, 1994; Giri et al., 1994; Kimura et al., 1995). Treatment of cells with IL-4 induces various biological responses, including an increase in cell proliferation and gene transcription. Some of these responses are unique to IL-4, and some are also induced by other cytokines. Although the receptors for IL-4 and IL-2 have several features in common, because of the γ chain receptor component, IL-4 evokes responses that IL-2 does not (Morla et al., 1988; Lin et al., 1995; Quelle et al., 1995).
Apoptotic cell death is a process that ultimately leads to activation of endogenous nucleases that promote internucleosomal DNA degradation (Wyllie, 1980). Apoptosis can be induced by growth factor deprivation (Duke and Cohen, 1986; Nuñez et al., 1990; Williams et al., 1990), signaling via surface receptors (Smith et al., 1989; Benhamou et al., 1990; Hasbold and Klaus 1990), and exposure to drugs (Zubiaga et al., 1992) or DNA-damaging agents (Waters, 1992). Cell surface receptor-mediated mechanisms that control apoptosis often act through a signal transduction system involving the stimulation of the receptor, the activation of protein kinase and phosphatase cascades, and the release of second messengers to up-regulate or suppress the transcription of specific genes.
Many components of the machinery that regulate and execute programmed cell death have been identified (Nagata et al., 1997). In addition to the central role of the caspase family of proteases and the Bcl-2 family of apoptosis regulators, recent reports have suggested that c-Jun N-terminal kinases (JNK) may be involved in controlling apoptosis (Kyriakis and Avruch, 1996).
JNKs are activated by stimuli such as UV light (Dèrijard et al., 1994), γ-irradiation (Chen et al., 1995; Kharbanda et al., 1995), protein synthesis inhibitors (Kyriakis et al., 1994), ceramide (Westwick et al., 1995), and DNA-damaging drugs (Van Dam et al., 1995; Yu et al., 1996), TNF and IL-1 (Sluss et al., 1994; Raingeaud et al., 1995). JNK activity is also induced by mitogenic signals, including growth factors (Hibi et al., 1993; Minden et al., 1994), oncogenic Ras (Dèrijard et al., 1994), CD40 ligation (Sakata et al., 1995; Berberich et al., 1996), and T cell activation signaling (Chen et al., 1996; Su et al., 1994). Included among JNKs are the 46-kDa JNK1 and the 55-kDa JNK2 isoforms (Hibi et al., 1993; Kyriakis et al., 1994), which phosphorylate transcription factors such as c-Jun, ATF-2, and Elk-1 (Gupta et al., 1995; Karin, 1995; Whitmarsch et al., 1995). JNK activation requires phosphorylation at the Thr and Tyr residues by the dual-specificity kinases SEK1 or MKK4, which are activated following phosphorylation. One potential function of JNKs appears to be the initiation of programmed cell death, although JNK pathway activation does not necessarily lead to apoptosis; IL-3, erythropoietin, and thrombopoietin activate the Ras/MEKK1/SEK1/JNK pathway without induction of apoptosis (Nagata et al., 1997).
The serine and threonine kinase c-Akt, also known as Racα or protein kinase Bα (Bellacosa et al., 1991; Coffer and Woodgett, 1991; Jones et al., 1991), is the cellular homologue of the v-Akt oncogene. Akt is composed of an N-terminal pleckstrin homology domain, followed by a catalytic domain and a short C-terminal tail. Akt is regulated by both phosphorylation and the direct binding of PI3 kinase (PI3K) lipid products to the pleckstrin homology domain of Akt (Konishi et al., 1996). In addition, other PI3K-independent mechanisms of Akt activation have been identified (Didichenko et al., 1996; Franke et al., 1997; Klippel et al., 1997). Akt is a mediator of growth factor-induced survival and suppresses the apoptotic death of a number of cell types induced by a variety of stimuli, including IL-2 and IL-3 deprivation, cell-cycle discordance, loss of cell adhesion, and DNA damage (Ahmed et al., 1997; Dudek et al., 1997; Kauffmann-Zeh et al., 1997; Kennedy et al., 1997; Khwaja et al., 1997; Kulik et al., 1997; Songyang et al., 1997).
In this study, we examined the role of Akt and JNK2 in apoptosis mediated by IL-4 deprivation of TS1αβ cells, and the relevance of these findings is discussed in the context of apoptosis.
MATERIALS AND METHODS
Cells and Cultures
TS1αβ is a lymphokine-dependent mouse T cell line stably transfected with the α and β chains of the human IL-2R (Pitton et al., 1993). The TS1αβ double transfectant responds independently to IL-2, IL-4, or IL-9. Cells were cultured in RPMI 1640 (Bio-Whittaker, Verviers, Belgium) supplemented with 5% heat-inactivated FCS (Life Technologies, Paisley, UK), 2 mM glutamine, 10 mM HEPES, 0.55 mM arginine, 0.24 mM asparagine, 50 μM 2-mercaptoethanol, and 60 U/ml IL-4 or 5 ng/ml recombinant IL-2.
Lymphokines, Antibodies, Reagents, and Plasmids
Human recombinant IL-2 was provided by Roussel Uclaf (Paris, France). Murine recombinant IL-4 or cultured supernatant of a HeLa subline (H28) transfected with the pKCRIL-4.neo plasmid was used as a source of murine IL-4. Mouse antipan Ras monoclonal antibody (Ab3) was obtained from Oncogene Science (Cambridge, MA). Rabbit polyclonal anti-JNK1 and -JNK2 were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-Akt has been previously described (Franke et al., 1995). Monoclonal anti-p85 PI3K, -Bcl-2, -Bax, and -Bcl-x antibodies were from Transduction Laboratories (Lexington, KY). Peroxidase (PO)-conjugated goat anti-rabbit or -mouse immunoglobulin antibody was provided by Dako (Glostrup, Denmark). Wortmannin and histone H2B were from Sigma Chemical Co. (St. Louis, MO), agarose-conjugated human c-Jun-GST was obtained from Upstate Biotechnolgy (Lake Placid, NY) and DEAE-dextran was from Pharmacia (Uppsala, Sweden). Enhanced chemiluminiscence (ECL) reagent and γ32P were provided by Amersham (Buckinghamshire, UK), and Capture-Tec pHook-3 kit was obtained from Invitrogen (San Diego, CA). The annexin-FITC kit was from Immunotech (Marseille, France).
Generation of dominant negative Akt (Akt K179 M), constitutively active Akt (Myr-Akt), and wild-type (wt) Akt have been previously described (Franke et al., 1995). In brief, constitutively activated myr-Akt.HA was generated by addition of a N-terminal src myristoylation signal to a construct encoding Akt.HA.
Transient Transfection
Transfections were performed using the DEAE-dextran method. Cells in exponential growth (1 × 107) were washed in TS buffer (25 mM Tris HCl, pH 7.4, 137 mM NaCl, 5 mM KCl, 0.7 mM CaCl, 0.5 mM MgCl2, and 0.6 mM Na2HPO4) and resuspended in 0.5 mg/ml DEAE-dextran in TS buffer containing 5 μg of the corresponding plasmid and pHook-3. A total of 6.75 ml RPMI with 5% FCS were added after 20 min incubation at room temperature. Cells were incubated for 1 h at 37°C, centrifuged, and resuspended in 10 ml RPMI with 5% FCS alone or supplemented with 60 U/ml IL-4. The capture-Tec pHook-3 kit was used for isolation of transiently transfected cells from a mixed population of transfected and nontransfected cells.
Cell Cycle Analysis
Propidium Iodide Staining.
A total of 2 × 105 cells were washed and resuspended in PBS, permeabilized with 0.1% NP-40, and stained with 50 μg/ml propidium iodide immediately before analysis. Samples were analyzed using an Epics XL flow cytometer (Coulter, Miami, FL). Apoptosis was measured as the percentage of cells present in the subG1 region of the fluorescence scale having a hypodiploid DNA content.
Annexin Staining.
A total of 4 × 105 cells were washed with ice-cold PBS, diluted in ice-cold binding buffer, and stained with annexin and propidium iodide. Samples were maintained on ice for 10 min in the dark and then analyzed by flow cytometry.
In Vitro JNK Assay
Cells (1 × 107) were lysed in radioimmunoprecipitation assay buffer (50 mM Tris HCl, pH 7.4, 1% NP-40, 0.25% Na deoxycholate, 150 mM NaCl, 1 mM EGTA, and protease inhibitors). Supernatants were immunoprecipitated with anti-JNK1 or -JNK2 antibody, followed by incubation with protein-A Sepharose beads. Immunoprecipitates were washed, mixed with 3 μg agarose-conjugated purified c-Jun-GST and 20 μM [γ-32P]ATP in 30 μl kinase buffer (20 mM HEPES, pH 7.6, 20 mM MgCl2, 100 μM sodium orthovanadate, and 20 mM β-glycerophosphate), and incubated at 30°C for 20 min. The reaction was stopped with SDS-PAGE sample buffer and boiled for 5 min before loading onto a 10% acrylamide gel. The gel was dried and exposed to x-ray film.
In Vitro Akt Kinase Assay
Cells (1 × 107) were lysed in lysis buffer (50 mM Tris HCl, pH 7.5, 1% NP-40, 150 mM NaCl, and 5 mM EDTA) with protease inhibitors for 20 min at 4°C and centrifuged (13,000 rpm for 15 min at 4°C). Supernatants were immunoprecipitated with anti-Akt antibody and protein-A Sepharose beads were then added. Immunoprecipitates were washed and the kinase assay carried in 30 μl Akt kinase buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2, and 10 mM MnCl2) containing 10 μCi [γ-32P]ATP, 5 μM unlabeled ATP, and 1 mM DTT. Histone H2B was added as exogenous substrate at a final concentration of 0.01 mg/ml. After 20 min at room temperature, the kinase assay was terminated by the addition of SDS-PAGE sample buffer and boiled for 5 min before loading onto a 12.5% gel. The gel was dried and exposed to x-ray film.
In Vitro PI3K Assay
Cells (1 × 107) were lysed in lysis buffer (137 mM NaCl, 20 mM Tris HCl, pH 8, 1 mM MgCl2, 1 mM CaCl2, 1% NP-40, and 10% glycerol) with protease and phosphatase inhibitors for 20 min at 4°C and centrifuged (13,000 rpm for 10 min at 4°C). Supernatants were precleared with protein-A Sepharose beads and immunoprecipitated with anti-p85 PI3K antibody. Protein-A Sepharose was added for 1 h at 4°C. Immunoprecipitates were then washed once with PBS; once with 0.5 M LiCl and Tris HCl, pH 7.4; once with H2O; and once with 10 mM Tris HCl, pH 7.4, 100 mM NaCl, and 1 mM EDTA. The kinase assay was performed in a final volume of 50 μl with 15 μCi [γ-32P]ATP, S-adenosine, and 20 μg l-α-phosphatidylinositol for 20 min at 24°C and terminated with 100 μl 1 M HCl. The lipids were extracted with 200 μl chloroform/methanol (1:1), washed with 80 μl methanol/1 M HCl (1:1), and separated by thin-layer chromatography on silica gel 60 plates coated with 1% potassium oxalate. Plates were developed in chloroform/methanol/4 M NH4OH (9:7:2) and exposed to x-ray film.
Western Blot
Cells (2 × 106) were lysed in Laemmli sample buffer and protein extracts separated by SDS-PAGE, transferred to nitrocellulose membrane, blocked with 5% nonfat dry milk in TBS (20 mM Tris HCl, pH 7.5, 150 mM NaCl), and incubated with the primary antibody in TBS/0.5% nonfat dry milk. Membranes were washed with 0.05% Tween-20 in TBS and incubated with a PO-coupled second antibody. After washing, labeled proteins were developed using the ECL system.
When stripping of blots was required, membranes were incubated with 62.5 mM Tris HCl, pH 6.8, 2% SDS, and 0.1 M 2-mercaptoethanol for 1 h at 56°C and washed extensively with TBS before reblocking and probing.
RESULTS
Apoptosis in IL-4-deprived TS1αβ Cells Correlates with Up-regulation of JNK2 Expression
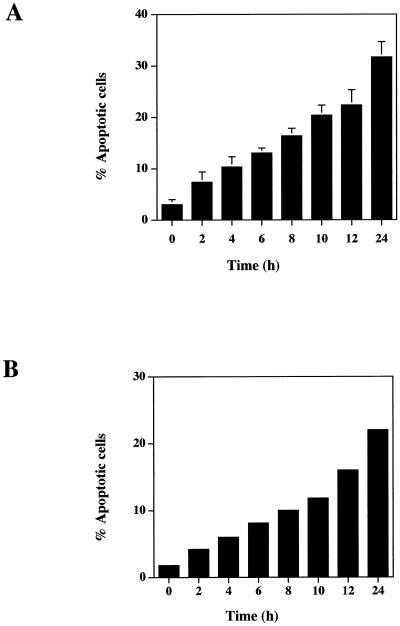
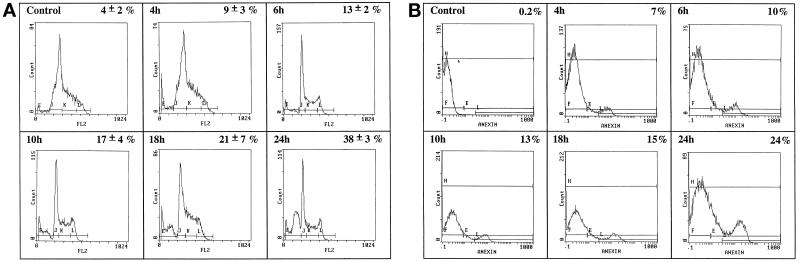
TS1αβ cells proliferate in the presence of IL-2, IL-4, or IL-9. When IL-4-maintained cells are deprived of lymphokine, they undergo apoptosis as estimated by flow cytometry (Figure 1A). As soon as 2 h after IL-4 withdrawal, 6% of cells were apoptotic, reaching ∼32% at 24 h, whereas control cells maintained in the presence of IL-4 showed no apoptosis. Similar results were obtained when the percentage of apoptotic cells was estimated by annexin staining (Figure 1B). The commitment point for apoptosis was 4–6 h (our unpublished results). It was thus of interest to determine whether induction of apoptosis by IL-4 deprivation could modify the expression of JNKs, apoptosis-related molecules, and Akt kinase, a molecule that is involved in apoptosis prevention as well as to Bcl-2.
Figure 1.
IL-4 deprivation induces apoptosis of TS1αβ cells. (A) Cells (2 × 105) were cultured in the presence or absence of IL-4 (60 U/ml) for 24 h. At different times, cells were harvested and analyzed for the percentage of apoptosis. Cells were permeabilized, stained with propidium iodide, and analyzed for DNA staining using fluorescence flow cytometry. The zero time point corresponds to control cells cultured in IL-4. Error bars represent ± SD from three independent experiments. Apoptosis was measured as cells present in the subG1 region of the fluorescence scale and having a hypodiploid DNA content. (B) Cells were treated as in A, diluted in ice-cold binding buffer, stained with annexin and propidium iodide, and maintained on ice for 10 min in the dark. Samples were analyzed by flow cytometry.
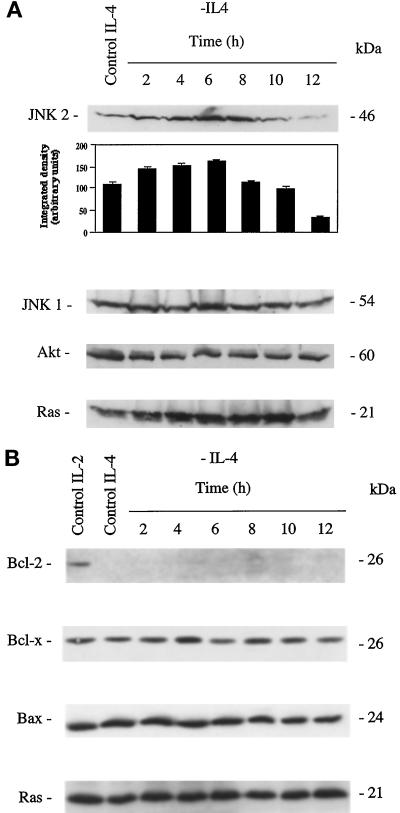
The protein level of JNK1, JNK2, and Akt after IL-4 deprivation of TS1αβ cells is shown in Figure 2A. JNK1 expression showed no difference throughout the starvation period analyzed compared with IL-4-stimulated control cells. Similarly, control IL-4-cultured or -deprived cells showed constant Akt expression throughout the starvation period analyzed. Interestingly, JNK2 expression was up-regulated after IL-4 deprivation; maximum expression was observed 4–8 h after IL-4 withdrawal, and disappeared almost completely 12 h after deprivation. Unaltered Ras expression was shown for all conditions as an internal protein loading control. These results show that in TS1αβ cells, IL-4 deprivation modifies JNK2 expression, whereas JNK1 and Akt expression are unaffected.
Figure 2.
Time course of JNK1, JNK2, Akt, Ras, Bcl-2, Bcl-x, and Bax expression in IL-4-deprived cells. (A) Control or IL-4-deprived cells (2 × 106) were harvested at different time points, lysed, and protein extracts separated in SDS-PAGE. Proteins were transferred to nitrocellulose and probed sequentially with anti-JNK1, -JNK2, -Akt, and -Ras antibody, the last as an internal protein loading control. Protein bands were detected using the ECL system. Similar results were obtained in three independent experiments. Molecular mass markers are indicated. Densitometric analysis of JNK2 expression is shown below the blot. (B) TS1αβ cells were stimulated by IL-4 or deprived of lymphokine. At different time points, cells (2 × 106) were lysed and protein extracts separated, transferred to nitrocellulose, and probed with anti-Bcl-2, -Bax, and -Bcl-x. Cells maintained in IL-2 were used as a positive control. Protein bands were detected using the ECL system. An internal control of homogeneous Ras expression in all samples is shown. Similar results were obtained in three independent experiments.
The TS1αβ cell line expressed the antiapoptotic protein Bcl-2 upon IL-2 stimulation, whereas IL-4-mediated growth proceeds in the absence of Bcl-2 (Figure 2B) and Bax expression (our unpublished results). Bcl-x and Bax levels were unaltered throughout the IL-4 starvation period analyzed. This suggests that IL-4 deprivation-induced apoptosis proceeds along pathways that do not involve changes in Bcl-x or Bax expression.
IL-4 Deprivation-induced Apoptosis Correlates with Reduction of Akt and PI3K and Induction of JNK2 Activity
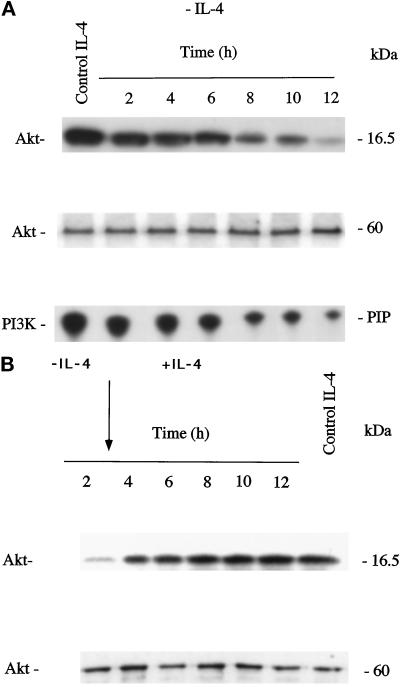
To examine the possible involvement of Akt, PI3K, and JNKs in IL-4 deprivation-induced apoptosis, we measured JNK1, JNK2, PI3K, and Akt kinase activity in IL-4-stimulated and IL-4-deprived TS1αβ cells. Akt was immunoprecipitated with a specific antibody at various time points after IL-4 deprivation, and the kinase activity in the immunoprecipitates was measured using [γ-32P]ATP and H2B as substrate. Akt activity was detected in IL-4-cultured control cells. Down-regulation of Akt activity was time dependent; 2 h after lymphokine deprivation, Akt activity was inhibited and was almost undetectable 12 h after IL-4 withdrawal (Figure 3A). To ensure equal protein loading, Akt activity was separated in SDS-PAGE, transferred to nitrocellulose, and probed with anti-Akt antibody.
Figure 3.
IL-4 deprivation down-regulates Akt and PI3K activity. (A) Cell lysates from control or IL-4-deprived cells (1 × 107) were immunoprecipitated with anti-Akt antibody, and the Akt immunocomplex assayed by phosphorylating H2B substrate in the presence of [γ-32P]ATP. Phosphorylated proteins were separated in SDS-PAGE and visualized by autoradiography. As an internal control of protein loading, Akt was separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-Akt antibody. Similar results were obtained in three independent experiments. Molecular mass markers are shown. PI3K activity was immunoprecipitated with anti-p85 PI3K antibody and determined by incorporation of [γ-32P]ATP into propidium iodide. Radiolabeled phosphatidylinositol phosphate (PIP) was analyzed by thin-layer chromatography on silica gel 60 plates and developed in chloroform/methanol/4 M NH4OH (9:7:2). Dried plates were exposed to x-ray film. Migration of PIP is indicated in the margin. Similar results were obtained in three independent experiments. (B) Cells were IL-4 deprived for 3 h and then IL-4 was added to the culture medium. Cell lysates from control, IL-4-deprived, or IL-4-restimulated cells (1 × 107) were immunoprecipitated with anti-Akt antibody and assayed as in A. Akt was separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-Akt antibody as internal control of protein loading. Molecular mass markers are shown.
PI3K activity was determined in α-p85 PI3K immunoprecipitates of control or IL-4-deprived cells (Figure 3A). PI3K activity was detected in IL-4-maintained cells, and decreased gradually throughout the IL-4 starvation period analyzed. The lowest level of activity was detected 12 h after deprivation.
Because Akt activity was reduced 2 h after IL-4 deprivation, it was of interest to know whether readdition of IL-4 after Akt activity down-regulation and before the commitment point would be able to restore Akt activity. Ts1αβ cells were deprived of IL-4 for 3 h and then IL-4 was added to the culture medium. Akt activity was immunoprecipitated before and after IL-4 readdition and the kinase activity measured using [γ-32P]ATP and H2B as substrate (Figure 3B). IL-4-cultured control cells showed Akt activity. Two hours after lymphokine deprivation, Akt activity was decreased. Interestingly, readdition of IL-4 after 3 h of starving restored Akt activity. As internal control of protein loading, Akt activity was separated in SDS-PAGE, transferred to nitrocellulose, and probed with anti-Akt antibody.
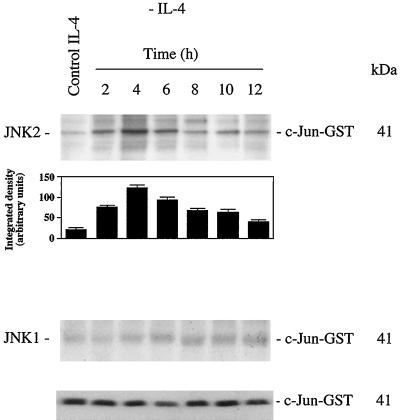
JNK was immunoprecipitated with anti-JNK1 or -JNK2-specific antibodies at various time points after IL-4 deprivation. The kinase activity in the immunoprecipitates was measured using [γ-32 P]ATP and purified c-jun-GST fusion protein. JNK1 activity was almost undetectable and remained constant throughout the IL-4 deprivation time course analyzed (Figure 4). A marked increase in JNK2 activity was detected as soon as 2 h after IL-4 deprivation, reaching a plateau at 4 h after starvation (Figure 4); kinase activity then decreases and basal activity was detected 12 h after IL-4 deprivation, as well as in control cells. As an internal protein loading control, JNK2 kinase activity was separated in SDS-PAGE, transferred to nitrocellulose, and probed with anti-c-Jun antibody. In addition, c-jun levels were not altered during the starvation period analyzed (our unpublished results).
Figure 4.
IL-4 deprivation induces JNK2 activation. Cell lysates from control or IL-4-deprived cells (1 × 107) were immunoprecipitated with anti-JNK1 or -JNK2 antibody. JNK1 and JNK2 immune complexes were assayed by phosphorylating the c-Jun-GST substrate in the presence of [γ-32P]ATP. Phosphorylated proteins were separated in SDS-PAGE and visualized by autoradiography. Densitometric analysis of JNK2 activity is shown below the autoradiography. As an internal control of c-Jun-GST protein loading in the reaction, JNK2 kinase activity measured in the presence of [γ-32P]ATP was separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-c-Jun antibody. Similar results were obtained in three independent experiments. Molecular mass markers are shown.
Transient Expression of Constitutively Activated Akt Prevents IL-4 Withdrawal-mediated Apoptosis
Because IL-4 deprivation in TS1αβ cells correlates with inhibition of Akt activity and apoptosis, we hypothesized that transfection with a myr-Akt mutant or wt Akt may activate a rescue pathway for apoptosis mediated by IL-4 deprivation.
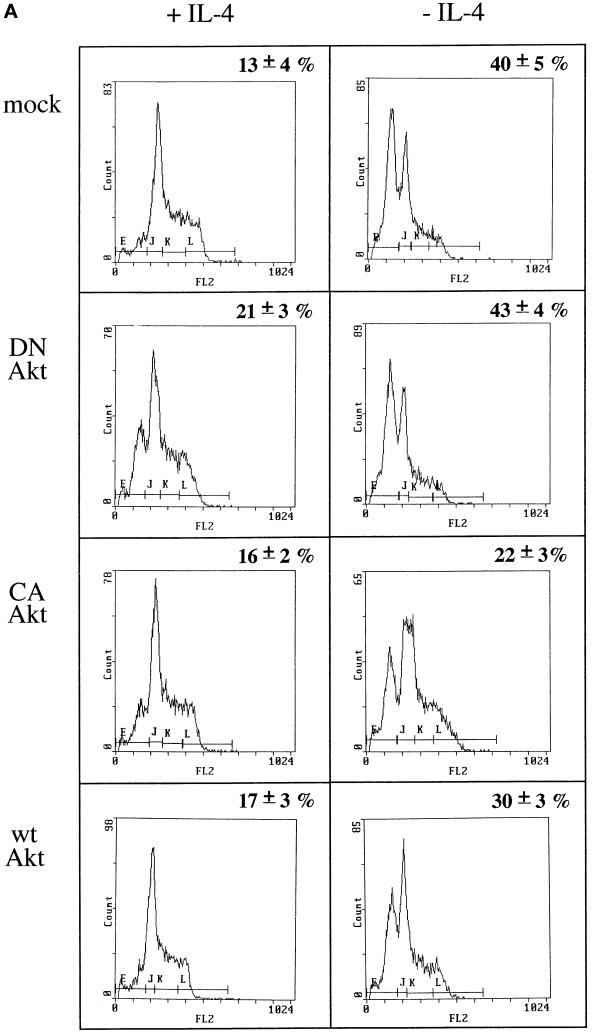
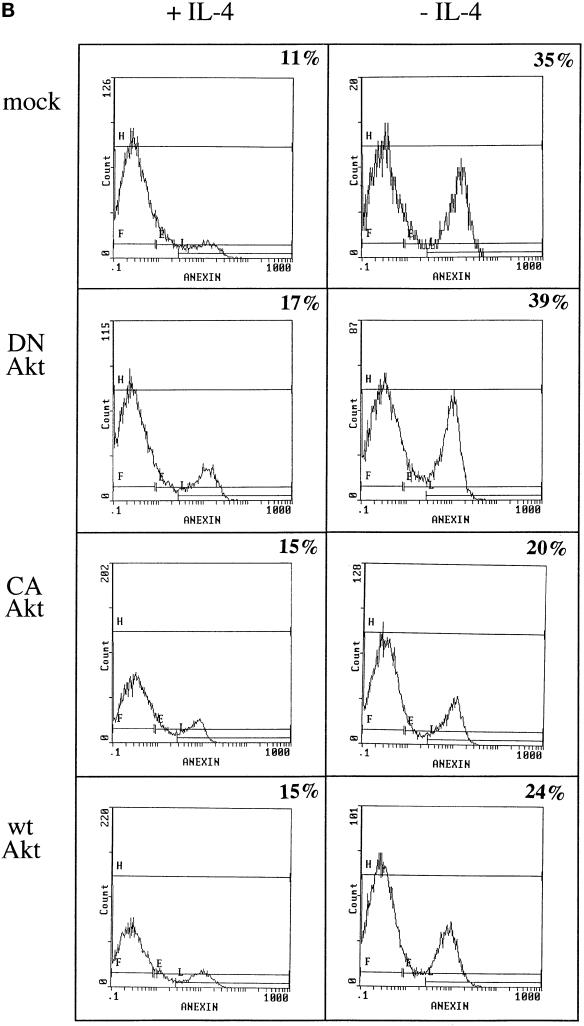
Cells transfected with myr-Akt, a constitutively activated mutant, and deprived of IL-4 showed a reduction of ∼45% in the fraction of apoptotic cells compared with IL-4-deprived mock-transfected control cells (Figure 5A. Similarly, when wt Akt-transfected cells were deprived of IL-4, the fraction of apoptotic cells was reduced to ∼25% of those observed in growth factor-deprived, mock-transfected cells. The apoptosis detected in cells transfected with the dominant negative Akt K179 M mutant in the absence of IL-4 was comparable with that seen in mock-transfected cells under the same culture conditions. The frequency of apoptotic cells remained ∼13–17% in all transfected cells in the presence of IL-4, except for Akt K179 M transfectants, which showed a slightly higher level of apoptosis, even in the presence of IL-4. Similar results were obtained when the percentage of apoptotic cells was estimated by annexin staining (Figure 5B).
Figure 5.
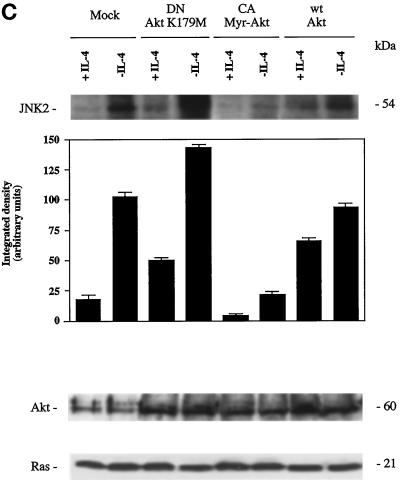
Overexpression of Akt induces JNK2 inhibition and prevents apoptosis induced by IL-4 deprivation in TS1αβ cells. (A) Cells were transfected with the indicated constructs by the DEAE-dextran method; maintained for 24 h in the presence or absence of IL-4; and then selected, washed, permeabilized, and stained with propidium iodide. Samples were analyzed by flow cytometry. The most proximal region of the fluorescence scale (region E) represents the subG1 region and corresponds to cells having a hypodiploid DNA content (apoptotic cells). The percentages of apoptotic cells in each sample are superimposed. Similar results were obtained in three independent experiments. (B) Cells were treated as in A. After selection, samples were diluted in ice-cold binding buffer, stained with annexin and propidium iodide, and analyzed by flow cytometry. Percentage of apoptotic cells is superimposed. (C) JNK2 activation and expression of wt Akt, Myr-Akt, or Akt K179 M in cells maintained in the presence or absence of IL-4. Cells were transiently transfected using the DEAE-dextran procedure and maintained 24 h after transfection without lymphokine or in the presence of IL-4. Cells were lysed and protein extracts immunoprecipitated with anti-JNK2 antibody and assayed for JNK2 activity as in Figure 4, or separated by SDS-PAGE, transferred to nitrocellulose, probed with anti-Akt antibody and developed using the ECL system. Densitometric analysis of JNK2 activity is shown below the autoradiography. The blot was stripped and probed with anti-Ras antibody as an internal control of protein loading. Similar results were obtained in three independent experiments. Molecular mass markers are shown.
It was of interest to determine whether constitutive expression of Akt could inhibit JNK2 activation shown after IL-4 deprivation. For this purpose, IL-4-deprived cells were transiently transfected with the constitutively activated myr-Akt mutant. Myr-Akt expression in IL-4-deprived cells strongly blocked JNK2 activation (Figure 5C); similarly, overexpression of wt Akt also significantly blocked JNK2 activation.
Expression of the transiently transfected Akt mutants in TS1αβ cells was confirmed by direct comparison of Akt protein levels in transfected cells and in mock-transfected controls (Figure 5C). Twenty-four h posttransfection, Akt expression was increased in IL-4-stimulated or IL-4-deprived cells transfected with myr-Akt, wt Akt, and Akt K179 M compared with mock transfectants. Unaltered Ras expression is shown as an internal control of protein loading.
Inhibition of PI3K Correlates with Reduction of Akt Activity and JNK2 Activation
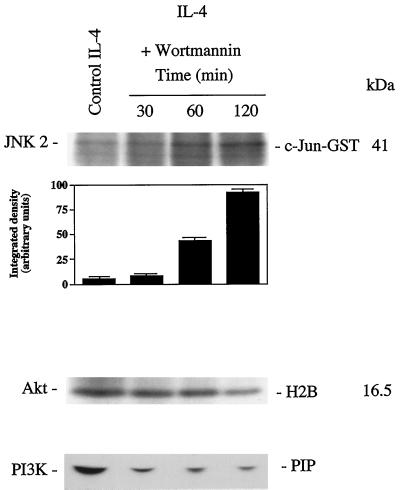
To test the role of PI3K in Akt activation and prevention of apoptosis, we inhibited PI3K activity using the inhibitor wortmannin. TS1αβ cells maintained in the presence of IL-4 were treated for different time periods with wortmannin. Treatment of cells induced a remarkable decrease in PI3K and Akt activity as compared with untreated control cells, and wortmannin treatment of IL-4 cultured cells increased JNK2 activity (Figure 6). Given that wortmannin induced reduction of Akt activity, it was of interest to analyze whether PI3K activity inhibition induced apoptosis. IL-4-maintained cells were treated with wortmannin for different time periods and apoptosis was analyzed. Apoptosis increased gradually with time, reaching 38% at 24 h (Figure 7A), which is similar to levels observed after IL-4 deprivation for a similar period of time (Figure 1). The percentage of apoptotic cells estimated by annexin staining was similar to that observed using propidium iodide staining (Figure 7B).
Figure 6.
Effect of wortmannin on PI3K, Akt, and JNK2 activity. Cell lysates from control or 100 nM wortmannin-treated cells were immunoprecipitated with anti-Akt or -JNK2 antibody. Akt or JNK2 immunocomplexes were assayed as described in Figures 3 and 4. Densitometric analysis of JNK2 activity is shown below the autoradiography. PI3K activity was determined as described in Figure 3. Migration of the PIP and molecular mass markers are indicated. Similar results were obtained in three independent experiments.
Figure 7.
Effect of the PI3K inhibitor wortmannin on the cell survival. (A) Cells cultured in the presence of IL-4 were supplemented with 100 nM wortmannin for different periods of time. After treatment, the percentage of apoptotic cells was analyzed. Cells were permeabilized, stained with propidium iodide, and analyzed for DNA staining by flow cytometry. The most proximal region of fluorescence, region E, represents the subG1 region, which corresponds to cells having a hypodiploid DNA content (apoptotic cells). Similar results were obtained in three independent experiments. (B) Cells were treated as in A. After washing, samples were diluted in ice-cold binding buffer, stained with annexin and propidium iodide, and analyzed by flow cytometry. Percentage of apoptotic cells is superimposed.
DISCUSSION
Using the lymphokine-dependent T cell line TS1αβ as a model, we analyzed the role of Akt and JNK in the process of cell death promoted by IL-4 deprivation. PI3K-dependent Akt activation is reduced in IL-4-deprived cells and readdition of IL-4 before the commitment point is able to restore Akt activity. Apoptosis induced by lymphokine withdrawal can be prevented by overexpression of Akt. In addition, IL-4 starvation correlates with induction of JNK2 activity.
Recent studies show that Akt activation by growth factors depends on D3 phosphoinositides (Ahmed et al., 1993; Burgering and Coffer 1995; Cross et al., 1995; Downward, 1995, Franke et al., 1995; Alessi et al., 1997; Stokoe et al., 1997), whose synthesis is catalyzed by PI3K (Datta et al., 1996; Klippel et al., 1996; Carpenter and Cantley, 1997). The PI3K inhibitor wortmannin inhibited IL-4-mediated protection from apoptosis in TS1αβ cells. Our results suggest that PI3K and Akt play a role in IL-4-mediated protection from apoptosis, because overexpression of a dominant negative Akt mutant triggers apoptosis, even in the presence of IL-4.
IL-3-dependent activation of Akt is mediated by PI3K activation; consistent with results showing that Akt promotes survival after IL-3 withdrawal (Songyang et al., 1997), Akt also promotes survival after IL-4 deprivation in TS1αβ cells. Myr Akt, which is constitutively activated independently of PI3K by localizing to the plasma membrane, reduces the level of IL-4 deprivation-induced apoptosis by ∼45%. wt aKT, which is activated by PI3K, is less effective in preventing apoptosis mediated by IL-4-deprivation, because PI3K activity is down-regulated in the absence of IL-4. In addition, IL-4 is able to restore Akt activity after 3 h of lymphokine deprivation. Akt is also important for IGF-I-dependent survival of primary cerebellar neurons (Dudek et al., 1997) and fibroblasts (Kulik et al., 1997).
Cells dependent on growth factor usually undergo apoptosis upon growth factor deprivation (Downward, 1995). Two major components play a role in the control of the cell death pathway: the Bcl-2 family members, which block apoptosis, and the interleukin convertin enzyme–like protease family, which executes the apoptotic pathway. In the case of IL-2, a signal transduced by Akt regulates Bcl-2 and c-Myc expression; as a result, these molecules inhibit apoptosis and stimulate proliferation (Ahmed et al., 1997). In TS1αβ cells, IL-4-mediated growth and inhibition of apoptosis proceed in the absence of Bcl-2 expression. Consistent with our results, IL-3-dependent Akt activation uses a mechanism for cell survival that does not involve Bcl-2 (Songyang et al., 1997). In addition, we detected no significant change in the steady-state levels of Bcl-x or Bax, either in IL-4-cultured cells or following lymphokine deprivation. IL-4 also induces protection from apoptosis in the myeloid progenitor cell line 32D and in murine spleen B cells via the insulin receptor substrate pathway (Zamorano et al., 1996). Finally, activation of the antiapoptotic signaling pathway PI3K/Akt protects fibroblasts from apoptosis induced by UV-B light and promotes survival of superior cervical neurons (Kulik et al., 1997; Philpott et al., 1997).
JNK and stress-activated protein kinase have been previously associated with the induction of apoptosis (Chen et al., 1995; Dèrijard et al., 1995; Van Dam et al., 1995; Yu et al., 1996). In our cell model, IL-4 deprivation-induced apoptosis correlates with JNK2 activation and up-regulation at the protein level. JNK activation does not necessarily lead to apoptosis; however, IL-1, IL-3, erythropoietin, and thrombopoietin also activate JNK and do not induce apoptosis (Nagata et al., 1997).
Akt activation by growth factors is at least partially dependent on Ras activation in some cell types (Franke et al., 1995), and coexpression of activated mutants of Ras stimulates Akt activity (Klippel et al., 1996; Kauffmann-Zeh et al., 1997). The ability of Ras to stimulate Akt is dependent on PI3K activation, placing this enzyme downstream of Ras in this signaling pathway. In IL-4-dependent T cell lines such as TS1αβ–Ras is not activated via the IL-4 receptor (Gómez et al., 1997).
Our results suggest that PI3K and Akt activity correlates with cell survival through the IL-4 receptor. PI3K and Akt inhibition caused by IL-4 deprivation or wortmannin treatment also correlates with JNK2 activation and the ability of Myr-Akt to mediate survival correlates with the inhibition of JNK2 activity.
Although many factor-dependent cell lines respond to IL-4 with increased [3H]thymidine incorporation into DNA, only a few cell lines have been successfully adapted to growth in IL-4 alone. Among them, TS1αβ and LD8, two murine T cell lines described by our group, can be propagated indefinitely in IL-4. The findings presented here using an IL-4-dependent murine T cell line as a cellular model, provide new and original insights into the mechanism by which IL-4 could regulate cell survival.
ACKNOWLEDGMENTS
We thank C. Mark for editorial assistance. The Department of Immunology and Oncology was founded and is supported by the Consejo Superior de Investigaciones Científicas and Pharmacia and Upjohn.
REFERENCES
- Ahmed NN, Franke F, Bellacosa A, Datta K, Gonzalez-Portal ME, Taguchi T, Testa JR, Tsichlis PN. The proteins encoded by c-akt and v-akt differ in post-translational modifications, subcellular localization and oncogenic potential. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p7056 kinase in vivo and in vitro. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, AKT, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:244–247. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Benhamou LE, Cazenave PA, Sarthou P. Anti-immunoglobulins induce death by apoptosis in WEHI-231 B lymphoma cells. Eur J Immunol. 1990;20:1405–1407. doi: 10.1002/eji.1830200630. [DOI] [PubMed] [Google Scholar]
- Berberich I, Shu G, Siebelt F, Woodgett JR, Kyriakis JM, Clark EA. Cross-linking CD40 on B cells preferentially induces stress-activated protein kinases rather than mitogen activated protein kinases. EMBO J. 1996;15:92–101. [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Pierce JH, Watson CJ, Falcon J, Ihle JN, Paul WE. B cell stimulatory factor-1/IL-4 mRNA is expressed by normal and transformed mas cells. Cell. 1987;50:809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatydilinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide kinases. Curr Opin Cell Biol. 1997;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- Chen Y-R, Meyer CF, Tan TH. Persistent activation of c-Jun N-terminal kinase (JNK1) in gamma radiation-induced apoptosis. J Biol Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- Chen YR, Meyer CF, Tan TH. Persistent activation of JNK1 in gamma irradiation-induced apoptosis. J Biol Chem. 1995;27:631–643. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Woodgett JR. Molecular-cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein-kinase-C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Cosman D. The hematopoietic receptor superfamily. Cytokine. 1993;5:95–106. doi: 10.1016/1043-4666(93)90047-9. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen sinthase kinase-3 by insulin mediated by PKB. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Datta K, Bellacosa A, Chan TO, Tsichlis PN. Akt is a direct target of the phosphatydilinositol 3 kinase. Activation by growth factor, v-src and v-Ha-ras in Sf9 and mammalian cells. J Biol Chem. 1996;271:30835–30839. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- Dèrijard B, Hibi M, Wu YH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Didichenko SA, Tilton B, Hemmings BA, Ballmer-Hofer K, Thelen M. Constitutive activation of the protein kinase B and phosphorylation of the p47phox by a membrane-targeted phosphoinositide 3-kinase. Curr Biol. 1996;6:1271–1278. doi: 10.1016/s0960-9822(02)70713-6. [DOI] [PubMed] [Google Scholar]
- Downward J. Signal transduction. A target for PI(3) kinase. Nature. 1995;376:553–554. doi: 10.1038/376553a0. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RS, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Duke RC, Cohen JJ. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SY, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the akt proto-oncogene is a target of the PDGF-activated phosphatydilinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumanki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J, Martínez-A. C, Fernandez B, Garcia A, Rebollo A. Ras activation leads to cell proliferation or apoptotic cell death upon IL-2 stimulation or lymphokine deprivation respectively. Eur J Immunol. 1997;27:1610–1618. doi: 10.1002/eji.1830270704. [DOI] [PubMed] [Google Scholar]
- Gupta S, Campbell D, Dèrijard B, Davis RJ. Transcription factor ATF2 regulation by JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Hasbold J, Klaus GGB. Anti-immunoglobulins antibodies induce apoptosis in immature B cell Lymphomas. Eur J Immunol. 1990;20:1685–1690. doi: 10.1002/eji.1830200810. [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Howard M, Farrar J, Hilfiker M, Johnson B, Takatsu K, Hanaoka T, Paul WE. Identification of a T cell derived beta cell growth factor distinct from IL-2. J Exp Med. 1982;155:914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular-cloning and identification of a serine threonine protein-kinase of the 2nd-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The regulation of AP1 activity by MAP kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of the c-Myc-induced apoptosis by Ras signaling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Ran R, Pandey P, Shafman TD, Feller SM, Weichselbaum RR, Kufe DW. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstorn S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Leonard WJ, Noguchi M, Russell SM. Sharing of a common gamma chain by the IL-4, IL-2 and IL-7 receptor. Adv Exp Med Biol. 1995;385:225–232. doi: 10.1007/978-1-4899-0987-9_23. [DOI] [PubMed] [Google Scholar]
- Klippel A, Kavanaugh WM, Pot D, Williams LT. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatydilinositol 3 kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Takeshita H, Higuchi M, Nakamura M, Sudo T, Nishikawa S, Sugamura K. Functional participation of the IL-2R gamma chain in IL-7 receptor complexes. Science. 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K, Sugamura K. Sharing the IL-2 receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Konishi K, Matsuzaki H, Tanaka M, Ono Y, Tokunaga C, Kuroda S, Kikkawa U. Activation of Rac-protein kinase by heat shock and hyperosmolarity stress through a pathway independent of PI3 kinase. Proc Natl Acad Sci USA. 1996;93:7639–7644. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubi EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of the c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lin J-X, et al. The role of pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13 and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, McMahon M, Lange-Carter C, Dèrijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- Morla AO, Schreurs J, Miyajima A, Wang JYJ. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988;8:2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Nishida E, Todokono K. Activation of JNK signaling pathway by erythropoietin, thrombopoietin and IL-3. Blood. 1997;89:2664–2669. [PubMed] [Google Scholar]
- Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. IL-2 receptor gamma chain: a functional component of the IL-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Nuñez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell line. J Immunol. 1990;144:3602–3610. [PubMed] [Google Scholar]
- Paul WE. IL-4: a multifunctional regulator of immunity and inflammation. J Cancer Res. 1991;82:1458–1459. [PubMed] [Google Scholar]
- Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated PI3 Kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139:809–815. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitton C, Rebollo A, Van Snick J, Theze J, García A. High-affinity and intermediate-affinity forms of the human interleukin 2 receptor, expressed in an interleukin 9-dependent murine T cell line, deliver proliferative signals via differences in their transduction pathways. Cytokine. 1993;5:362–371. doi: 10.1016/1043-4666(93)90069-h. [DOI] [PubMed] [Google Scholar]
- Quelle FW, et al. Cloning of murine Stat6, Stat proteins that are tyrosine phosphorylated in response to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995;15:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedman M, Berg M, McVicar DW, Witthunn BA, Silvennoinen O. Interaction of IL-2R beta and gamma chains with Jak1 and Jak3. implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann MC, Miyajima A, Puri RK, Paul WE. IL-2R gamma chain, a functional component of the IL-4 receptor. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Sakata N, Patel HR, Terada N, Aruffo A, Johnson GL, Gelfond EW. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD-40 on human B cells. J Biol Chem. 1995;270:30823–30828. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- Seder RA, Paul WE, Ben-Sasson SZ, LeGross GS, Kagey-Sobotka A, Finkelman FD, Pierce JH, Plant M. Production of IL-4 and other cytokines following stimulation of mas cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1981;94:137–140. doi: 10.1159/000235345. [DOI] [PubMed] [Google Scholar]
- Sluss HK, Barrett T, Dèrijard B, Davis RJ. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJT. Antibodies to CD3/T cell receptor complex induce cell death by apoptosis in immature T cell in thymic culture. Nature. 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol 3,4,5-triphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during co-stimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Van Dam H, Wilhem D, Herr I, Steffen A, Herlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RL. Radiation-induced apoptosis in a murine T-cell hybridoma. Cancer Res. 1992;52:883–890. [PubMed] [Google Scholar]
- Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270:22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- Whitmarsch AJ, Shore P, Sharroks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- Williams GT, Smith CA, Spooncer E, Drexter TM, Taylor DR. Haemopoietic colony-stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990;343:76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous nuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yu R, Shtil AA, Tan TH, Roninson IB, Kong ANT. Adriamicyn activates c-jun N-terminal kinase in human leukemia cells. A relevance to apoptosis. Cancer Lett. 1996;107:73–81. doi: 10.1016/0304-3835(96)04345-5. [DOI] [PubMed] [Google Scholar]
- Zamorano J, Wang HY, Wang LM, Pierce JH, Keegan AD. IL-4 protects cells from apoptosis via the insulin receptor substrate pathway and a second independent signaling pathway. J Immunol. 1996;157:4926–4934. [PubMed] [Google Scholar]
- Zubiaga AM, Muñoz E, Huber BT. IL-4 and IL-2 selectively rescue Th cell subsets from glucocorticoid-induced apoptosis. J Immunol. 1992;149:107–112. [PubMed] [Google Scholar]