Abstract
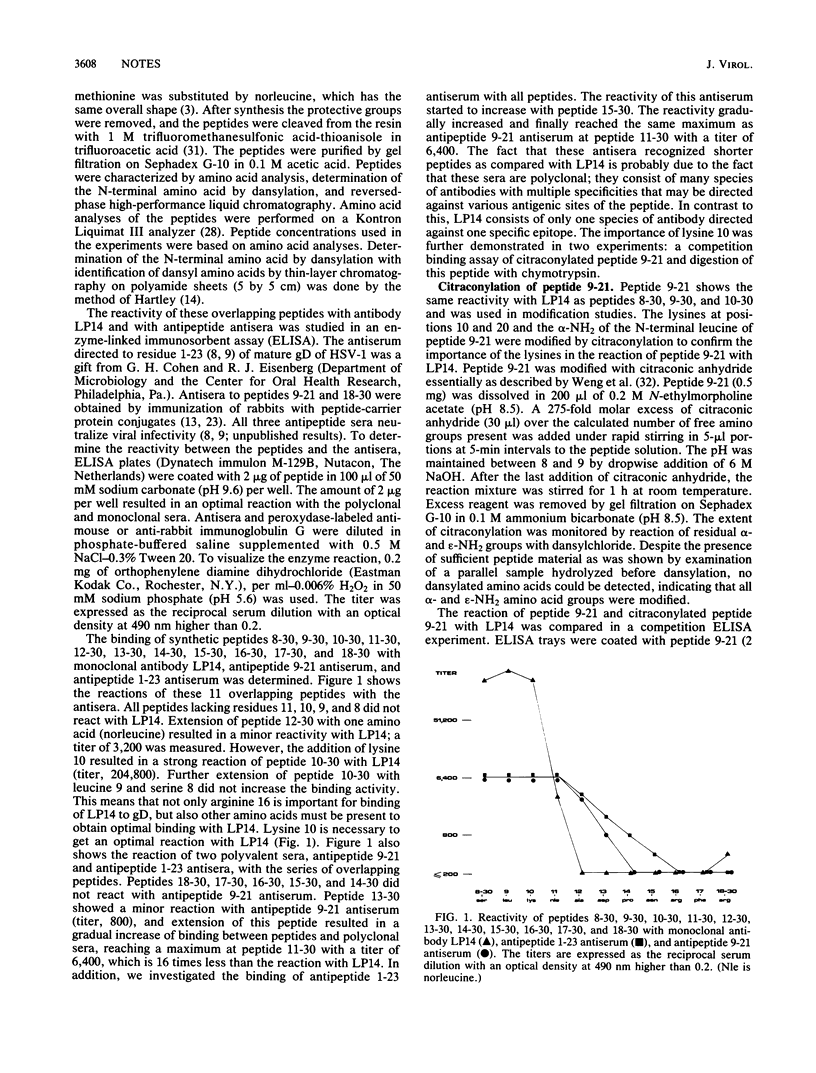
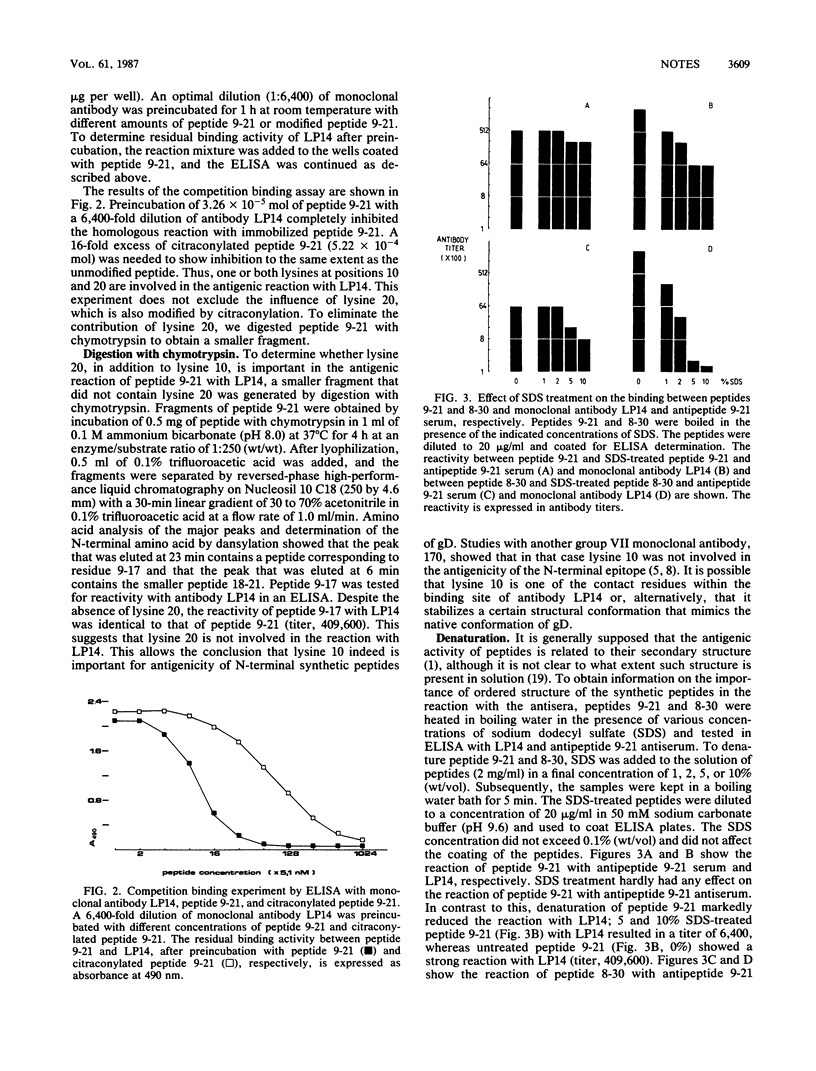
To investigate the contribution of individual amino acids to the antigenicity of the N-terminal region of herpes simplex virus type 1 glycoprotein D, a series of 14 overlapping synthetic peptides within residues 1 to 30 were examined for their reactivity with monoclonal antibody LP14 (a group VII monoclonal antibody; in herpes simplex virus mutants resistant to LP14, arginine 16 is substituted by histidine) and two antipeptide antisera (antipeptide 9-21 and antipeptide 1-23). Maximal binding was achieved with peptides 9-21, 10-30, 9-30, and 8-30 and the chymotryptic fragment 9-17 of peptide 9-21, suggesting that a major antigenic site is located within residues 10 through 17. Lysine 10 was shown to be essential for high reactivity, either by binding directly to the antibody molecule or by stabilizing an ordered structure of the peptide. The importance of ordered structure was demonstrated by a decrease in reactivity after sodium dodecyl sulfate treatment of peptides 9-21 and 8-30.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R. Chemically defined antiviral vaccines. Annu Rev Microbiol. 1980;34:593–618. doi: 10.1146/annurev.mi.34.100180.003113. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. L. Protective immunization of mice with specific HSV-1 glycoproteins. Immunology. 1983 Jun;49(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K. J., Mackett M., Wohlenberg C., Notkins A. L., Moss B. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science. 1985 May 10;228(4700):737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Eisenberg R. J., Ponce de Leon M., Golub E., Hudecz F., Varrichio A., Cohen G. H. Fine structure analysis of type-specific and type-common antigenic sites of herpes simplex virus glycoprotein D. J Virol. 1984 Nov;52(2):431–435. doi: 10.1128/jvi.52.2.431-435.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Cerini C. P., Heilman C. J., Joseph A. D., Dietzschold B., Golub E., Long D., Ponce de Leon M., Cohen G. H. Synthetic glycoprotein D-related peptides protect mice against herpes simplex virus challenge. J Virol. 1985 Dec;56(3):1014–1017. doi: 10.1128/jvi.56.3.1014-1017.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Pereira L., Hampar B., Zweig M., Cohen G. H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982 Feb;41(2):478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Ponce de Leon M., Matthews J. T., Spear P. G., Gibson M. G., Lasky L. A., Berman P., Golub E., Cohen G. H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985 Feb;53(2):634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984 Feb;43(2):761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Houghten R. A., Walker L. E., Reisfeld R. A., Wilson I. A., Hogle J. M., Lerner R. A. Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4949–4953. doi: 10.1073/pnas.80.16.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E., Mussgay M., Böhm H. O., Schulz G. E., Schaller H. Antibodies against a preselected peptide recognize and neutralize foot and mouth disease virus. EMBO J. 1982;1(7):869–874. doi: 10.1002/j.1460-2075.1982.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Type-specific delayed hypersensitivity and protective immunity induced by isolated herpes simplex virus glycoprotein. J Immunol. 1983 Mar;130(3):1413–1418. [PubMed] [Google Scholar]
- Simmons A., Nash A. A. Role of antibody in primary and recurrent herpes simplex virus infection. J Virol. 1985 Mar;53(3):944–948. doi: 10.1128/jvi.53.3.944-948.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereijken J. M., Hofsteenge J., Bak H. J., Beintema J. J. The amino-acid sequence of the three smallest CNBr peptides from p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Eur J Biochem. 1980 Dec;113(1):151–157. doi: 10.1111/j.1432-1033.1980.tb06149.x. [DOI] [PubMed] [Google Scholar]
- Weng L., Russell J., Heinrikson R. L. The covalent structure of bovine liver rhodanese. NH2-terminal sequence and partial structural analysis of tryptic peptides from the citraconylated protein. J Biol Chem. 1978 Nov 25;253(22):8093–8101. [PubMed] [Google Scholar]
- Williamson M. P., Hall M. J., Handa B. K. 1H-NMR assignment and secondary structure of a herpes simplex virus glycoprotein D-1 antigenic domain. Eur J Biochem. 1986 Aug 1;158(3):527–536. doi: 10.1111/j.1432-1033.1986.tb09786.x. [DOI] [PubMed] [Google Scholar]
- Williamson M. P., Handa B. K., Hall M. J. Secondary structure of a herpes simplex virus glycoprotein D antigenic domain. Int J Pept Protein Res. 1986 May;27(5):562–568. [PubMed] [Google Scholar]


