Abstract
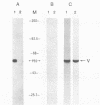
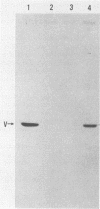
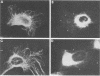
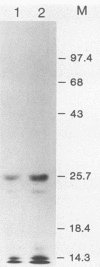
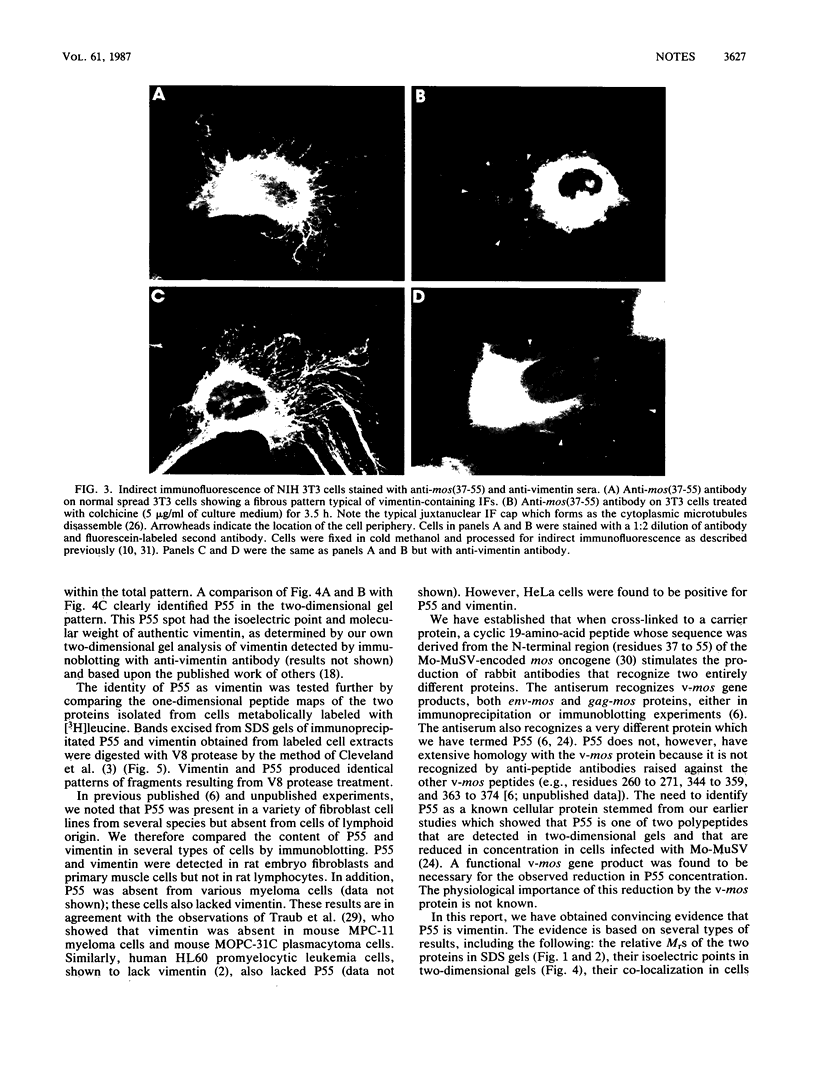
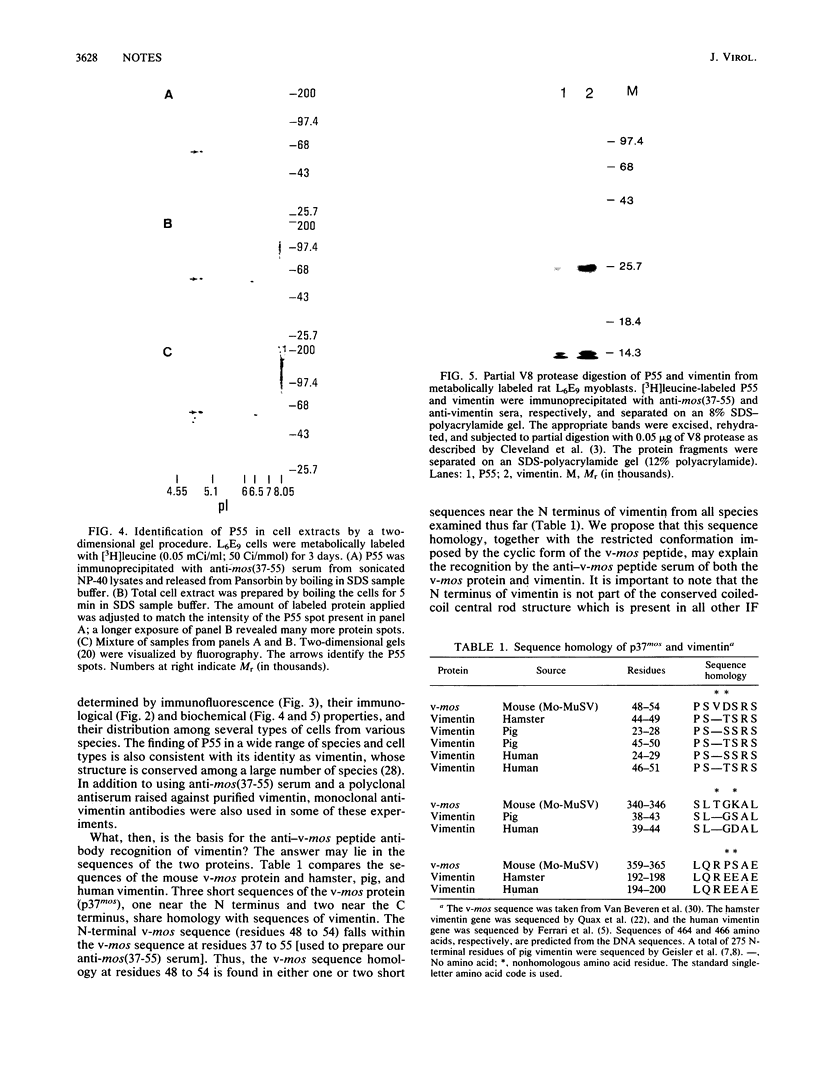
Rabbit antiserum prepared against a cyclic 19-amino-acid peptide predicted from the sequence of the viral mos gene (v-mos) of Moloney murine sarcoma virus not only recognized v-mos gene products but also specifically detected a 55,000-Mr polypeptide expressed in a variety of cells that grow on solid surfaces. This normal cellular protein, previously shown to be reduced in amount in cells expressing the v-mos gene, was found to be the intermediate filament structural protein, vimentin. This conclusion was reached by comparing relative mobilities in denaturing gels, isoelectric points, immunoreactivities, location in the cell, and peptide maps.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlinghaus R. B. The ts110 Moloney mouse sarcoma virus system: gag-mos gene products and cellular transformation. J Gen Virol. 1985 Sep;66(Pt 9):1845–1853. doi: 10.1099/0022-1317-66-9-1845. [DOI] [PubMed] [Google Scholar]
- Bernal S. D., Chen L. B. Induction of cytoskeleton-associated proteins during differentiation of human myeloid leukemic cell lines. Cancer Res. 1982 Dec;42(12):5106–5116. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Escobedo J., Singh B., Dina D., Arlinghaus R. B. Temperature-dependent cytocidal effects of Moloney murine sarcoma virus. Virus Res. 1986 Oct;6(1):75–84. doi: 10.1016/0168-1702(86)90058-4. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallick G. E., Sparrow J. T., Singh B., Maxwell S. A., Stanker L. H., Arlinghaus R. B. Recognition of mos-related proteins with an antiserum to a peptide of the v-mos gene product. J Gen Virol. 1985 May;66(Pt 5):945–955. doi: 10.1099/0022-1317-66-5-945. [DOI] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. Amino acid sequence characterization of mammalian vimentin, the mesenchymal intermediate filament protein. FEBS Lett. 1983 Oct 31;163(1):22–24. doi: 10.1016/0014-5793(83)81153-3. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4120–4123. doi: 10.1073/pnas.78.7.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Goldman A., Green K., Jones J., Lieska N., Yang H. Y. Intermediate filaments: possible functions as cytoskeletal connecting links between the nucleus and the cell surface. Ann N Y Acad Sci. 1985;455:1–17. doi: 10.1111/j.1749-6632.1985.tb50400.x. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Goldman A. E., Yang H. Y., Goldman R. D. The organizational fate of intermediate filament networks in two epithelial cell types during mitosis. J Cell Biol. 1985 Jan;100(1):93–102. doi: 10.1083/jcb.100.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. Further characterization of the P85gag-mos -associated protein kinase activity. Virology. 1984 Oct 15;138(1):143–155. doi: 10.1016/0042-6822(84)90154-5. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. A cAMP-independent serine/threonine kinase activity is associated with the mos sequences of ts110 Moloney murine sarcoma virus-encoded P85gag-mos. J Gen Virol. 1985 Oct;66(Pt 10):2135–2146. doi: 10.1099/0022-1317-66-10-2135. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. Serine kinase activity associated with Maloney murine sarcoma virus-124-encoded p37mos. Virology. 1985 May;143(1):321–333. doi: 10.1016/0042-6822(85)90119-9. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. Use of site-specific antipeptide antibodies to perturb the serine kinase catalytic activity of p37mos. J Virol. 1985 Sep;55(3):874–876. doi: 10.1128/jvi.55.3.874-876.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Purification of the intermediate filament protein vimentin from Ehrlich ascites tumor cells. J Biol Chem. 1982 May 25;257(10):5536–5543. [PubMed] [Google Scholar]
- Nigg E. A., Walter G., Singer S. J. On the nature of crossreactions observed with antibodies directed to defined epitopes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5939–5943. doi: 10.1073/pnas.79.19.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Quax W., Egberts W. V., Hendriks W., Quax-Jeuken Y., Bloemendal H. The structure of the vimentin gene. Cell. 1983 Nov;35(1):215–223. doi: 10.1016/0092-8674(83)90224-6. [DOI] [PubMed] [Google Scholar]
- Singh B., Hannink M., Donoghue D. J., Arlinghaus R. B. p37mos-associated serine/threonine protein kinase activity correlates with the cellular transformation function of v-mos. J Virol. 1986 Dec;60(3):1148–1152. doi: 10.1128/jvi.60.3.1148-1152.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Sparrow J. T., Hedge A. M., Arlinghaus R. B. Expression of the v-mos gene alters a Mr 55,000 protein during acute infection by Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3629–3633. doi: 10.1073/pnas.83.11.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Wittenberg C., Reed S. I., Arlinghaus R. B. Moloney murine sarcoma virus encoded p37mos expressed in yeast has protein kinase activity. Virology. 1986 Jul 30;152(2):502–506. doi: 10.1016/0042-6822(86)90156-x. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub U. E., Nelson W. J., Traub P. Polyacrylamide gel electrophoretic screening of mammalian cells cultured in vitro for the presence of the intermediate filament protein vimentin. J Cell Sci. 1983 Jul;62:129–147. doi: 10.1242/jcs.62.1.129. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Lieska N., Goldman A. E., Goldman R. D. A 300,000-mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J Cell Biol. 1985 Feb;100(2):620–631. doi: 10.1083/jcb.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff R. V., Goldman R. D. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6226–6230. doi: 10.1073/pnas.76.12.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]