Abstract
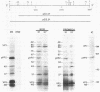
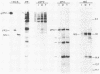
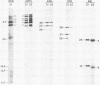
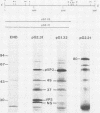
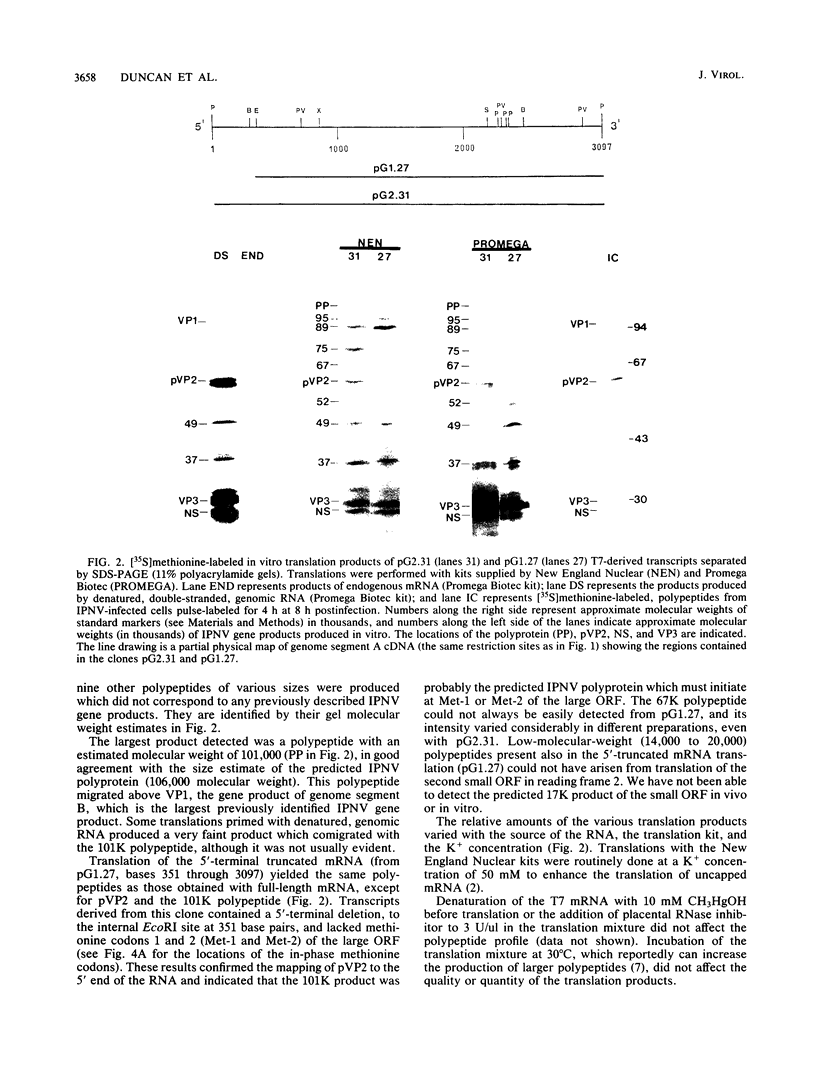
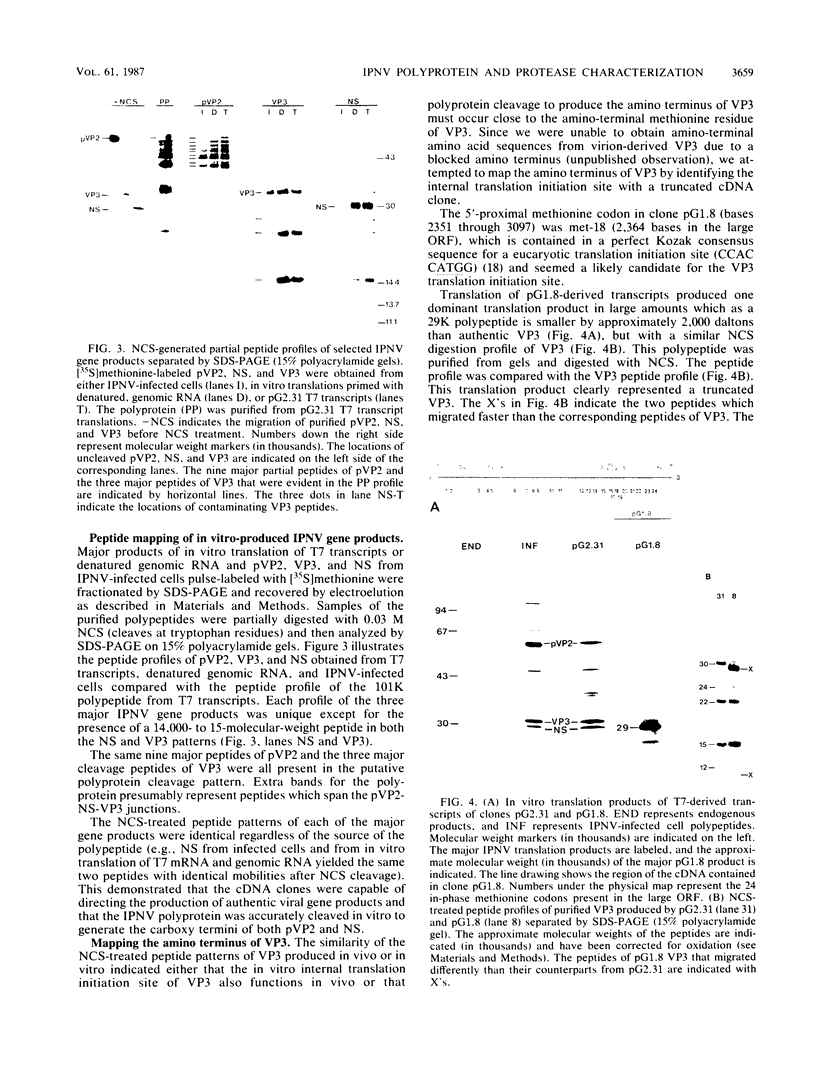
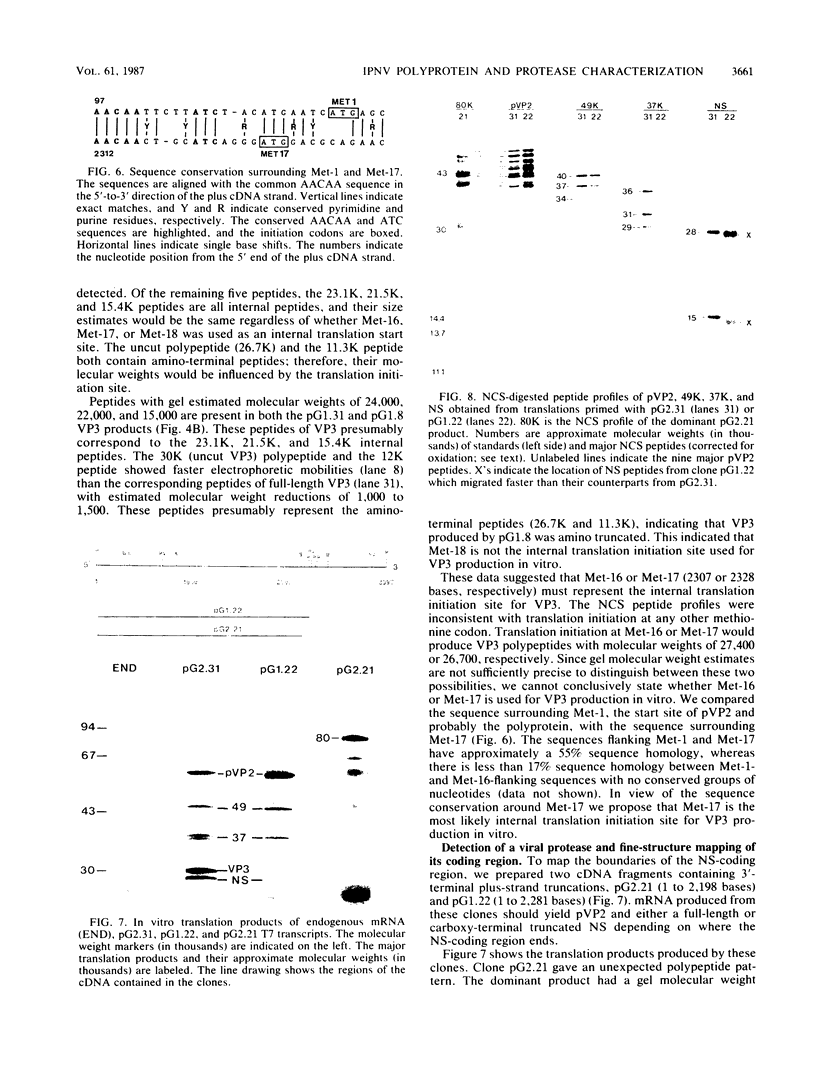
Full-length and truncated genome segment A-specific infectious pancreatic necrosis virus cDNA was subcloned into plasmid transcription vectors, and runoff transcripts were produced in vitro. These transcripts were translated in cell-free rabbit reticulocyte lysates and the translation products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Virus-specific polypeptides were gel purified and mapped by partial proteolysis with N-chlorosuccinimide and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Peptide profiles were compared with those of the corresponding polypeptides purified from infectious pancreatic necrosis virus-infected cells or prepared by in vitro translation of denatured genomic RNA. The cDNA directed the synthesis of authentic pVP2, VP3, and NS polypeptides as well as a number of previously undescribed polypeptides. A 101,000-molecular-weight polypeptide was isolated and shown to be the unprocessed infectious pancreatic necrosis virus polyprotein. The NS polypeptide appears to be a virus-encoded protease responsible for the cleavage of pVP2 from the polyprotein. The carboxy terminus of NS was mapped to within three or four amino acids on the polyprotein. The most likely internal translation start sites responsible for NS and VP3 production in vitro were also mapped.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azad A. A., Barrett S. A., Fahey K. J. The characterization and molecular cloning of the double-stranded RNA genome of an Australian strain of infectious bursal disease virus. Virology. 1985 May;143(1):35–44. doi: 10.1016/0042-6822(85)90094-7. [DOI] [PubMed] [Google Scholar]
- Bergmann J. E., Lodish H. F. Translation of capped and uncapped vesicular stomatitis virus and reovirus mRNA'S. Sensitivity to m7GpppAm and ionic conditions. J Biol Chem. 1979 Jan 25;254(2):459–468. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar L. W., Esparza J., Hudson G. R., Chmelo R., Lee P. W., Joklik W. K. Cloning the double-stranded RNA genes of reovirus: sequence of the cloned S2 gene. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7644–7648. doi: 10.1073/pnas.79.24.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. A., Richardson C., Kolakofsky D. Ribosomal initiation at alternate AUGs on the Sendai virus P/C mRNA. J Virol. 1986 Feb;57(2):684–687. doi: 10.1128/jvi.57.2.684-687.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dobos P., Hill B. J., Hallett R., Kells D. T., Becht H., Teninges D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979 Nov;32(2):593–605. doi: 10.1128/jvi.32.2.593-605.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Semler B. L., Jackson R. J., Hanecak R., Duprey E., Wimmer E. In vitro translation of poliovirus RNA: utilization of internal initiation sites in reticulocyte lysate. J Virol. 1984 May;50(2):507–514. doi: 10.1128/jvi.50.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Dobos P. The nucleotide sequence of infectious pancreatic necrosis virus (IPNV) dsRNA segment A reveals one large ORF encoding a precursor polyprotein. Nucleic Acids Res. 1986 Jul 25;14(14):5934–5934. doi: 10.1093/nar/14.14.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassin D., Korn R., Horwitz M. S. A major internal initiation site for the in vitro translation of the adenovirus DNA polymerase. Virology. 1986 Nov;155(1):214–224. doi: 10.1016/0042-6822(86)90181-9. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Internal initiation of translation on the vesicular stomatitis virus phosphoprotein mRNA yields a second protein. J Virol. 1986 Jun;58(3):797–804. doi: 10.1128/jvi.58.3.797-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. T., Manning D. S., Warner M., Stephens E. B., Leong J. C. A physical map of the viral genome for infectious pancreatic necrosis virus Sp: analysis of cell-free translation products derived from viral cDNA clones. J Virol. 1986 Dec;60(3):1002–1011. doi: 10.1128/jvi.60.3.1002-1011.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson P. J., McKern N. M., Power B. E., Azad A. A. Genomic structure of the large RNA segment of infectious bursal disease virus. Nucleic Acids Res. 1986 Jun 25;14(12):5001–5012. doi: 10.1093/nar/14.12.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Sung M. T. Use of N-chlorosuccinimide/urea for the selective cleavage of tryptophanyl peptide bonds in proteins. Cytochrome c. J Biol Chem. 1977 Jul 25;252(14):4976–4980. [PubMed] [Google Scholar]
- Macdonald R. D., Dobos P. Identification of the proteins encoded by each genome segment of infectious pancreatic necrosis virus. Virology. 1981 Oct 30;114(2):414–422. doi: 10.1016/0042-6822(81)90222-1. [DOI] [PubMed] [Google Scholar]
- Mertens P. P., Dobos P. Messenger RNA of infectious pancreatic necrosis virus is polycistronic. Nature. 1982 May 20;297(5863):243–246. doi: 10.1038/297243a0. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Conrad B. Microcomputer programs for graphic analysis of nucleic acid and protein sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):811–817. doi: 10.1093/nar/12.1part2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E., Duncan R., Krell P., Dobos P. Mapping of the large RNA genome segment of infectious pancreatic necrosis virus by hybrid arrested translation. Virology. 1987 May;158(1):211–217. doi: 10.1016/0042-6822(87)90255-8. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Emmert A. Modulation of the expression of poliovirus proteins in reticulocyte lysates. Virology. 1986 Jan 30;148(2):255–267. doi: 10.1016/0042-6822(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sargan D. R., Gregory S. P., Butterworth P. H. A possible novel interaction between the 3'-end of 18 S ribosomal RNA and the 5'-leader sequence of many eukaryotic messenger RNAs. FEBS Lett. 1982 Oct 18;147(2):133–136. doi: 10.1016/0014-5793(82)81026-0. [DOI] [PubMed] [Google Scholar]
- Schwindinger W. F., Warner J. R. DNA sequence analysis on the IBM-PC. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):601–604. doi: 10.1093/nar/12.1part2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]