Abstract
Cell–cell interactions, mediated by members of the cadherin family of Ca2+-dependent adhesion molecules, play key roles in morphogenetic processes as well as in the transduction of long-range growth and differentiation signals. In muscle differentiation cell adhesion is involved in both early stages of myogenic induction and in later stages of myoblast interaction and fusion. In this study we have explored the involvement of a specific cadherin, namely N-cadherin, in myogenic differentiation. For that purpose we have treated different established lines of cultured myoblasts with beads coated with N-cadherin–specific ligands, including a recombinant N-cadherin extracellular domain, and anti-N-cadherin antibodies. Immunofluorescent labeling for cadherins and catenins indicated that treatment with the cadherin-reactive beads for several hours enhances the assembly of cell–cell adherens-type junctions. Moreover, immunofluorescence and immunoblotting analyses indicated that treatment with the beads for 12–24 h induces myogenin expression and growth arrest, which are largely independent of cell plating density. Upon longer incubation with the beads (2–3 d) a major facilitation in the expression of several muscle-specific sarcomeric proteins and in cell fusion into myotubes was observed. These results suggest that surface clustering or immobilization of N-cadherin can directly trigger signaling events, which promote the activation of a myogenic differentiation program.
INTRODUCTION
Intercellular adhesion plays key roles in tissue formation and in the transduction of transmembrane signals affecting cell growth, motility, and differentiation. One of the most prominent and widespread groups of adhesion molecules involved in such interactions is the cadherin family, whose members mediate homophilic and Ca2+-dependent cell–cell adhesion in a wide variety of tissues (for review, see Geiger and Ayalon, 1992; Overduin et al., 1995; Shapiro et al., 1995; Takeichi, 1995). Cadherins are transmembrane molecules that interact with similar cadherins on neighboring cells via their ectodomains and with the actin-based cytoskeleton via their cytoplasmic regions. In addition, it has recently been established that a variety of signaling molecules are associated with cadherin-containing junctions, including receptor tyrosine kinases and cytoplasmic kinases of src family (Geiger et al, 1990, 1995; Yamada and Geiger, 1997). This colocalization suggested that accumulation of these molecules in junctional sites may lead to their activation and to adhesion-mediated signaling. Interestingly, the deterioration of these complexes as a result of cadherin or vinculin deficiency (Rodriguez Fernandez et al., 1993; Birchmeier, 1995; Volberg et al., 1995) or extensive tyrosine phosphorylation (Volberg et al., 1992; Ayalon and Geiger, 1997) is commonly found in malignant cells, leading to their anaplastic morphology and deregulated growth (Tsukita et al., 1993; Birchmeier and Behrens, 1994; Birchmeier, 1995). Recent evidence also indicates that junctional proteins, such as β-catenin and plakoglobin, can play a critical role in regulating cell fate not just by controlling the assembly of adherens junctions but also by directly activating transcription in the nucleus (Barth et al., 1997). Cadherin-mediated adhesion is also indirectly implicated in the differentiation of various cell types, including muscle cells (Knudsen, 1990; Knudsen et al., 1990; Zeschingk et al., 1995), chondrocytes (Oberlender and Tuan, 1994), osteoclasts (Mbalaviele et al., 1995), and neural cells (Doherty and Walsh, 1994).
Myogenesis is a particularly appealing system to study the role of cadherin-mediated adhesion in cell differentiation, because Ca2+-dependent cell adhesion, followed by cell fusion, is an intrinsic step in the differentiation process. The differentiation of cultured skeletal myoblasts is commonly activated by growth factor withdrawal and accompanied by transcriptional activation of muscle-specific genes, growth arrest, and fusion to form multinucleated myotubes (Olson, 1992, 1993). The muscle-specific basic helix–loop–helix transcription factors, including MyoD, Myf5, myogenin, and Mrf4, orchestrate the entire expression program of the various muscle-specific genes.
Several lines of indirect evidence suggest that cadherin-mediated interactions are also involved in the regulation of skeletal myogenesis. These include the inhibition of fusion by calcium depletion or by anti-N-cadherin antibodies and HAV-containing inhibitory peptide (Knudsen et al., 1990; Mege et al., 1992). In addition, the somites formed in N-cadherin knockout mice are small and irregularly shaped (Radice et al., 1997). In addition to the effect of N-cadherin on terminal stages of skeletal muscle differentiation, it has been shown to affect the expression of genes before the cell fusion stage: injection of a dominant negative cadherin RNA suppresses the expression of MyoD in Xenopus embryos and affects the subsequent expression of muscle-specific genes (Holt et al., 1994). Avian embryonic progenitor cells expressing only N-cadherin and not E-cadherin differentiate into skeletal muscle, and treatment with anti-N-cadherin antibodies inhibits the accumulation of myosin in chick embryo cells derived from different stages of avian embryonic development (George-Weinstein et al., 1997). Overexpression of N-cadherin in baby hamster kidney cells stimulates expression of sarcomeric myosin in these cells (Redfield et al., 1997). However, although these data suggest that cadherin-mediated interactions are involved in muscle differentiation, they do not indicate whether they are involved merely in the promotion of myoblast–myoblast adhesion per se or also induce long-range, myogenic signals that promote muscle gene expression.
In the present study we examined the effect of direct long-range signaling induced by N-cadherin clustering or immobilization on myogenic differentiation. We show here that beads conjugated to different N-cadherin ligands can trigger myogenesis, manifested by accelerated myoblast adhesion, myogenin expression, formation and assembly of various structural sarcomeric components, and myoblast fusion. Stimulation of myogenin expression by the N-cadherin–reactive beads occurred irrespective of myoblast density, suggesting that activation of this key step in myogenesis is directly induced by N-cadherin signaling.
MATERIALS AND METHODS
Cell Culture
All myoblast lines examined in this study, including C2 mouse skeletal myoblasts and L8 and L84 rat skeletal myoblasts, were kindly provided by Dr. D. Yaffe (The Weizmann Institute of Science) (Yaffe and Saxel, 1976, 1977). The cells were cultured in subconfluent densities at 37°C in a humidified atmosphere containing 8% CO2 in dishes coated with 0.1% gelatin. C2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% heat-inactivated FCS (BioLabs, Israel), glutamine, and antibiotics. L8 and L84 cells were cultured in Waymouth’s medium containing 15% FCS. Myogenic differentiation of L8 and L84 cells was induced by changing the growth medium to DMEM containing 2% heat-inactivated horse serum (Biological Industries, Israel) and 4 IU/ml insulin (Humulin R; Lilly, France). To trigger the differentiation of C2 myoblasts, cells were either plated at high density or stimulated by insulin and 10% horse serum in DMEM.
Preparation and Application of Cadherin-reactive Beads
N-cadherin ectodomain (NEC) was produced as described by Levenberg et al. (1998a). Briefly, 108 latex Polybead amino microsperes (mean diameter, 6 μm; Polysciences, Warrington, PA) were washed with phosphate-buffered saline (PBS; pH 7.4), activated overnight with 8% glutaraldehyde, washed with PBS, and incubated for 5 h with 500 μg/ml bovine serum albumin (BSA; Sigma Chemical, St. Louis, MO), purified NEC (Levenberg et al., 1998), or anti-N-cadherin monoclonal antibodies (clone BE; Volk and Geiger, 1986). Free sites were blocked with 0.5 M ethanolamine for 30 min, followed by incubation with 10 mg/ml BSA for 30 min, and the beads were resuspended in storage buffer (PBS containing 10 mg/ml BSA, 0.1% sodium azide, and 5% glycerol, pH 7.4). Aliquots containing 5 × 105 beads were added to cell monolayers in 35-mm-diameter culture dishes.
Cytochemical Staining
Myoblasts were cultured on 35-mm tissue culture dishes (Falcon, Becton Dickinson, Palo Alto, CA), coated with 0.1% gelatin, washed twice in PBS, and fixed for 10 min with methanol at room temperature. The monolayer was washed twice with PBS and stained for 25 min with 10% Giemsa solution (Fluka, Buchs, Switzerland), extensively washed with water, and dry mounted for microscopic examination.
Immunochemical Reagents and Procedures
Myoblasts cultured on glass coverslips coated with 0.1% gelatin were washed with 0.1 M 4-morpholinepropanesulfonic acid buffer (pH 6.0), permeabilized for 2 min by 0.5% Triton X-100 in 0.1 M 4-morpholinepropanesulfonic acid buffer, and fixed for 25 min with 3% paraformaldehyde in PBS. All of these procedures were carried out at room temperature. Anti-skeletal α-actin (5C5), anti-skeletal α-actinin (EA53), anti-skeletal myosin (MY32), anti-desmin (DEU10), and anti-pan-cadherin (CH19) were purchased from Sigma. Anti-β-catenin (94.5) was a gift from Dr. M. Wheelock (University of Toledo, Toledo, OH). Anti-titin (T12) and anti-myomesin (BB78) were obtained from Dr. W. Obermann and Dr. D. Fürst (Max-Plank-Institut for Biophysical Chemistry, Gottingen, Germany). Anti-myogenin antibodies were obtained from Dr. Barbara Winter and Dr. H. Arnold (Technical University, Braunschweig, Germany). Anti-5-bromo-2′-deoxyuridine (BrdU) was purchased from Becton Dickinson. For BrdU labeling cells were incubated for 45 min with 10 μM BrdU (Sigma) in culture medium, fixed, permeabilized for 4 min with 0.5% Triton X-100 in 3% paraformaldehyde, and post-fixed for 25 min with 3% paraformaldehyde. For anti-BrdU and 4′,6-diamidino-2-phenylinodole (DAPI, Sigma) labeling, the cells were treated with 2 M HCl in 0.5% Triton X-100 for an additional 15 min. The secondary antibodies were Cy-3-conjugated goat anti-mouse immunoglobulin (Jackson ImmunoResearch Laboratories, West Grove, PA). Nuclei were indirectly immunolabeled and counterstained by 10 min incubation with 2.5 μg/ml DAPI, and the cells were mounted in Elvanol (Mowiol 4-88; Hoechst, Frankfurt, Germany). Immunofluorescence microscopy was carried out with an Axiophot microscope (Zeiss, Oberkochen, Germany) equipped for multiple fluorescence examination.
Immunoblot Analysis
Whole cells were washed with PBS and extracted with Laemmli sample buffer. Proteins were separated by 10% SDS-PAGE (Laemmli, 1970) and transferred by electroblotting to Hybond-C nitrocellulose membranes (Amersham, Buckinghamshire, United Kingdom). Membranes were blocked for 1 h with a 4% solution of dry milk in PBS and then incubated overnight at 4°C with the primary antibodies diluted in PBS. After washing in PBS, the membranes were incubated for 45 min at room temperature with HRP-conjugated goat anti-mouse immunoglobulin G (Amersham), and immunoreactive bands were visualized using the Enhanced Chemiluminiscence system (Amersham).
Transmission Electron Microscopy
C2 cells were plated overnight on gelatin-coated 35-mm dishes. After 48 h of treatment with beads the cells were fixed in Kranovsky’s fixative (3% paraformaldehyde, 2% glutaraldehyde, 5 mM CaCl2, and 0.1 M sucrose in 0.1 M cacodylate buffer, pH 7.4) and post-fixed with 1% osmium tetroxide, 0.5% potassium dichromate, and 0.5% potassium hexacyanoferrate in 0.1 M cacodylate buffer. The cells were stained en bloc with 2% aqueous uranyl acetate, followed by ethanol dehydration. The dishes were embedded in Epon 812 (Tuosimis, MD). Sections were cut using a diamond knife (Diatome, Biel, Switzerland) and examined using a Philips (Mahwah, NJ) CM-12 transmission electron microscope operating at an accelerating voltage of 100 kV.
RESULTS
Interactions of Cadherin-reactive Beads with Cultured Myoblasts
To test the effect of N-cadherin–mediated interactions on myogenic differentiation, established myoblast cell lines were treated with 6-μm beads, coated with N-cadherin ligands (NEC or anti-N-cadherin monoclonal antibodies [BE]), as described by Levenberg et al. (1998a). BSA-coated beads were used as controls.
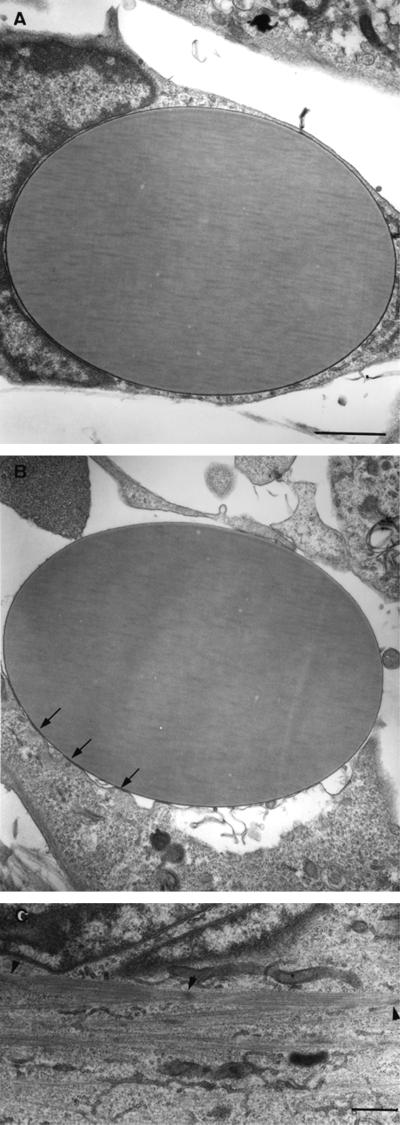
Transmission electron microscopy of C2 myoblasts after 48 h of incubation with the beads, coated either with NEC (Figure 1B) or with BSA (Figure 1A) indicated that both types of beads attach firmly to the cell surface. BSA-coated beads attached to the plasma membrane via a continuos close contact area and were engulfed by the cells after several hours of incubation, whereas the NEC-coated beads, were attached to the cells through electron-dense “foot-like processes,” resembling focal contacts, and were usually not extensively engulfed.
Figure 1.
Transmission electron micrographs of C2 myoblasts treated with beads coated with BSA (A) or NEC (B and C). The cells were incubated with the beads for 48 h in growth medium, fixed, and processed for transmission electron microscopy. Notice that the contact region with the NEC-coated bead is characterized by focal, foot-like adhesions (arrows), whereas the attachment to the BSA beads is tight and uniform. Sarcomeric filament bundles were found in the cytoplasm of the N-cadherin–stimulated cells (C) (arrowheads point to Z lines). Bars, 1 μm.
Promotion of Myotube Formation by N-Cadherin–mediated Stimulation
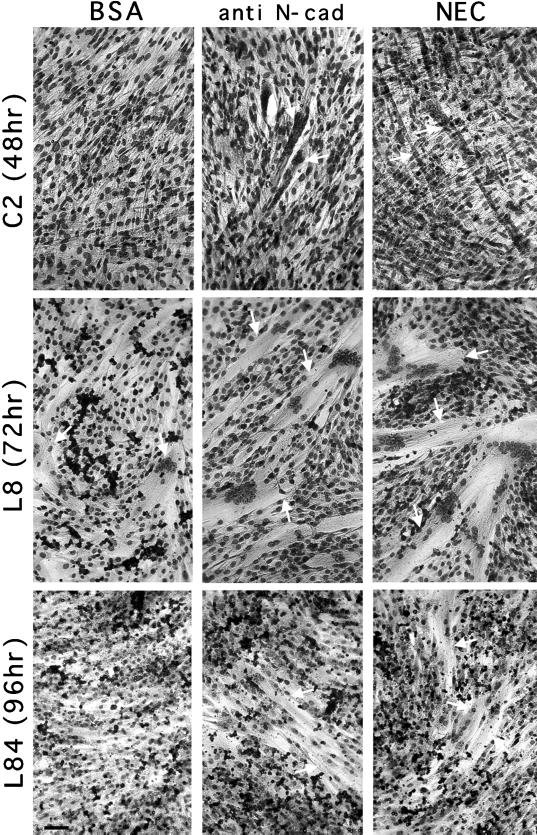
To test the direct involvement of N-cadherin–mediated signaling in skeletal muscle differentiation, we have examined the effects of N-cadherin–reactive and control beads on the rate of myotube formation by the different myogenic cell lines under conditions that do not favor differentiation (i.e., high serum concentration and low plating density). As demonstrated in Figure 2, the binding of cadherin-reactive beads (beads coated with NEC or with anti N-cadherin antibodies) to the cells significantly increased the number of myotubes in these myogenic cultures from ∼4/mm2 (BSA-coated beads) to 7 or 8/mm2 (BE- and NEC-coated beads, respectively). It is noteworthy that myotubes formed after treatment with cadherin-reactive beads were usually larger than those formed after treatment with control beads. The number of nuclei per individual tube was, however, variable, usually displaying clusters of 5–20 nuclei, and apparently did not depend on the type or number of bound beads.
Figure 2.
Effect of the N-cadherin stimulation on myotube formation in C2, L8, and L84 myogenic cell lines. C2, L8, and L84 myoblasts were treated with NEC and anti-N-cadherin (anti N-cad) BE beads for the indicated periods, fixed, and stained with Giemsa. C2 cells were maintained in growth medium, whereas for L8 and L84 cells the culture medium was replaced with differentiation medium simultaneously with the addition of beads. Media were changed every second day. Notice that the number of myotubes in the field is higher for the cells treated with cadherin-reactive beads. Arrows point to some of the multinucleated myotubes. Bar, 100 μm.
Transmission electron microscopy of C2 cells, after 48 h treatment with cadherin-reactive beads, revealed scattered sarcomeric structures in the cytoplasm (Figure 1C), which could be found in essentially all sections. These sarcomers were similar to those formed later in the course of differentiation induced by growth factors deprivation. Such organized filaments were not detected at that time point in >30 sections derived from C2 cells treated with control beads.
Expression and Assembly of Sarcomeric Components Induced by Cadherin-reactive Beads
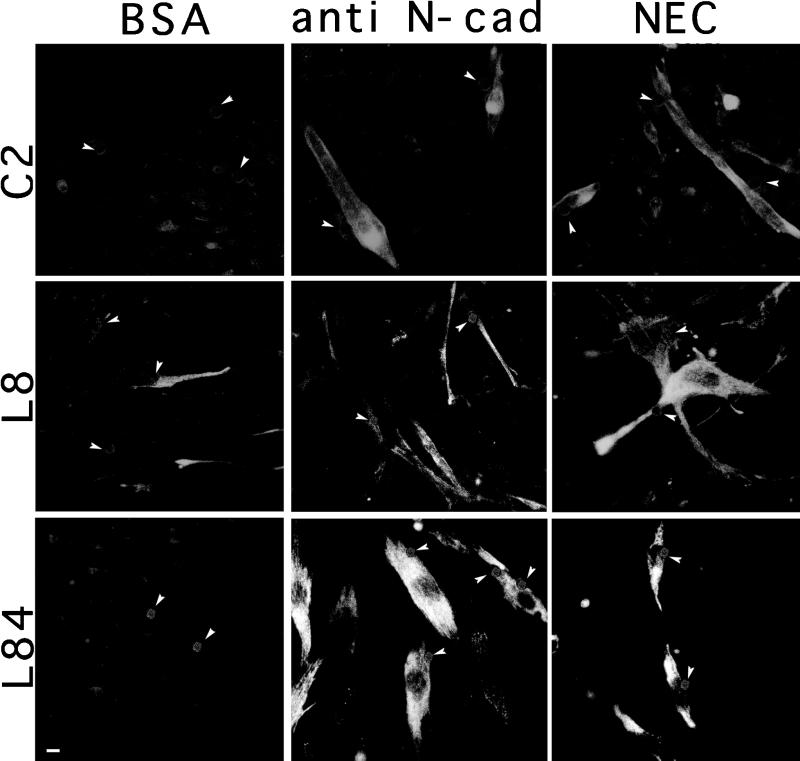
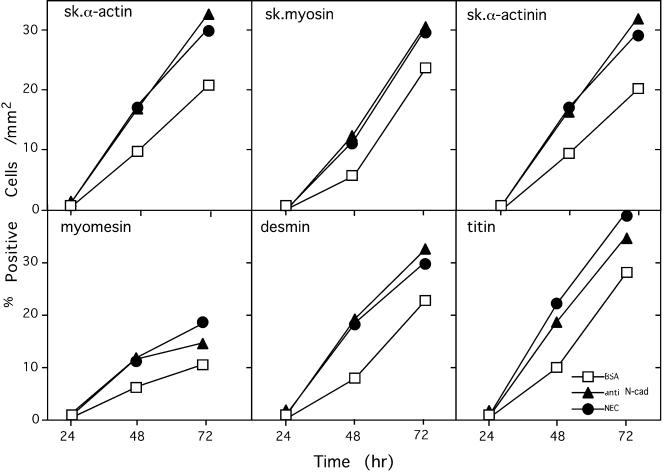
Expression of sarcomeric constituents is an essential element of the myogenic program and serves as a common phenotypic marker for muscle differentiation (Andrés and Walsh, 1996). We have thus examined the expression and assembly of different sarcomeric constituents in C2, L8, and L84 myoblasts after treatment with the various beads and found that cadherin stimulation of the three cell lines increases the number of cells expressing different muscle structural proteins, including skeletal muscle myosin, myomesin, skeletal α-actin, titin, skeletal α-actinin, and M-protein. An example showing a typical increase in the number of cells expressing skeletal myosin in myoblasts following such treatment is presented in Figure 3. The increase in the number of C2 cells expressing several muscle proteins was quantified after treatment with the different beads and is summarized in Figure 4. As shown, the various muscle proteins are first detected in control cells ∼48 h after plating, and the numbers increase progressively upon longer incubation. Addition of cadherin-reactive beads to these cells nearly doubles the number of cells expressing the various muscle proteins at 48 h and maintains a higher number of positive cells also at 72 h (Figure 4). We further determined the number of positive cells and overall levels of skeletal α-actinin in cultures treated with the various beads. As shown in Figure 5, the number of α-actinin–positive cells associated with cadherin-reactive beads is significantly higher than in control cultures, and, similarly, the total level of skeletal α-actinin is elevated (Figure 5B). It is noteworthy that because not all the cells in the culture were physically associated with beads, the actual increase induced by the cadherin ligands is probably even higher.
Figure 3.
Expression of skeletal myosin in myocytes treated with beads conjugated to NEC, anti-N-cadherin BE antibodies (anti N-cad) or BSA. C2, L8, and L84 myoblasts were treated with different beads for 48 h, permeabilized, fixed, and immunostained with anti-skeletal myosin antibodies. C2 cells were maintained in growth medium, whereas for L8 and L84 cells growth medium was replaced with differentiation medium simultaneously with the addition of beads. The number of cells per field was approximately equal. Notice the increase in myosin expression in the cultures after treatment with the cadherin-reactive beads. The position of individual beads was detected by phase-contrast microscopy, and their location is indicated by arrowheads. Bar, 10 μm.
Figure 4.
Effect of cadherin-reactive beads on the expression of various sarcomeric components in C2 cells. C2 myoblasts were plated overnight on gelatin-coated coverslips at 20% confluence, and beads coated with BSA or with the cadherin ligands were added. After additional incubation for 48 h after application of beads, cells were permeabilized, fixed, and labeled for the various muscle proteins. The number of positive cells in 10 randomly chosen microscopic fields (with 40× objective) was determined for each sample at every time point.
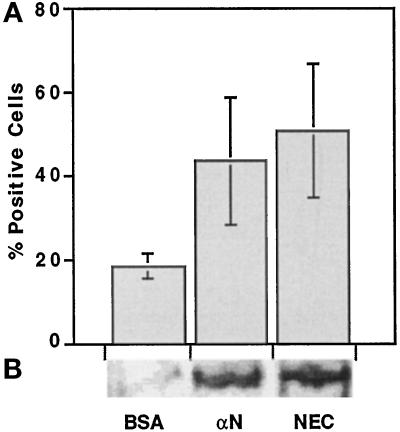
Figure 5.
Effect of cadherin-reactive beads on the expression of skeletal α-actinin in C2 cells. C2 myoblasts were plated and cultured overnight on gelatin-coated coverslips (A) or tissue culture dishes (B) at 50% confluence. Beads were added for 48 h, and then the samples were either fixed and immunostained or subjected to protein extraction and SDS-PAGE immunoblot analysis with antibodies against skeletal α-actinin. (A) Calculation of the percentage of α-actinin–positive, bead-bound cells. Each column represents the mean ± SD of 4 independent experiments; 200 cells were counted in each case. (B) Representative immunoblot analysis of the total cell extracts prepared from C2 cells treated as in A. αN, anti-N-cadherin antibodies.
Effects of N-Cadherin Stimulation on Cell Cycle Progression in C2 Myoblasts
Proliferation and differentiation of skeletal myoblasts are mutually exclusive processes, and cell cycle arrest is a prerequisite for activation of muscle-specific gene expression (Andrés and Walsh, 1996; Olson, 1992). To examine the effect of cadherin-reactive beads on the cell cycle, bead-treated C2 cells were pulsed with BrdU and immunofluorescently labeled with anti-BrdU antibodies. As shown in Figure 6, treatment of C2 myoblasts with beads coated with anti-N-cadherin antibodies suppresses the entry of cells into S phase compared with control BSA-coated beads. Application of beads coated with NEC induces a similar inhibition of cell proliferation (our unpublished results).
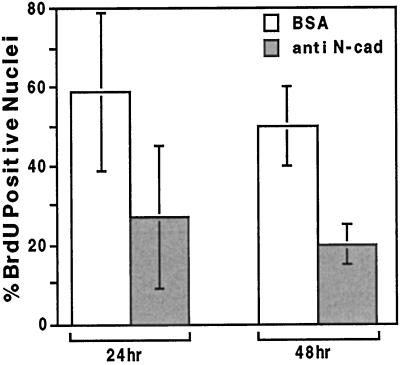
Figure 6.
Inhibition of S-phase entry in C2 myoblasts treated with N-cadherin–reactive beads. C2 myoblasts were plated and cultured overnight on gelatin-coated coverslips at 50% confluence. Cells were incubated with the various beads for 24 or 48 h and pulsed for 45 min with BrdU, fixed, and stained with anti-BrdU antibodies. Nuclei were visualized by DAPI, and the percentage of BrdU-positive nuclei was calculated. Each column represents the mean ± SD of 3 independent experiments; 1500 cells were counted in each case. Analysis of the differences between the cells treated with anti-N-cadherin and control beads was examined using Student’s t test (one tailed, paired) pointed to a significant decrease in the percentage of cells in S phase after 24 h of treatment (p = 0.002) and especially after 48 h of treatment (p = 0.0017).
Stimulation of Myogenin Expression in Myoblasts Treated with Cadherin-reactive Beads
Skeletal muscle differentiation is driven and coordinated by the expression of myogenic transcription factors, such as MyoD, Myf5, myogenin, and Mrf4 (Olson and Klein, 1994; Yun and Wold, 1996). In the established myoblast lines used here, MyoD and Myf5 are already present before differentiation is induced, and myogenin transcription is up-regulated upon myogenic induction (Olson and Klein, 1994). Because myogenin activity is crucial for the activation of the entire differentiation program, we have checked whether its expression is affected by N-cadherin stimulation, using both immunocytochemical (Figures 7A and 8) and Western blotting (Figures 7B and 9A) approaches. Both assays revealed a major increase in the expression of myogenin in cells treated with cadherin-reactive beads. Densitometric evaluation indicated a twofold increase in myogenin levels in cadherin bead–treated C2 cells. In L8 and L84 cells the increase was three- and fivefold, respectively. This increase is similar to the increase in the incidence of myogenin-positive nuclei.
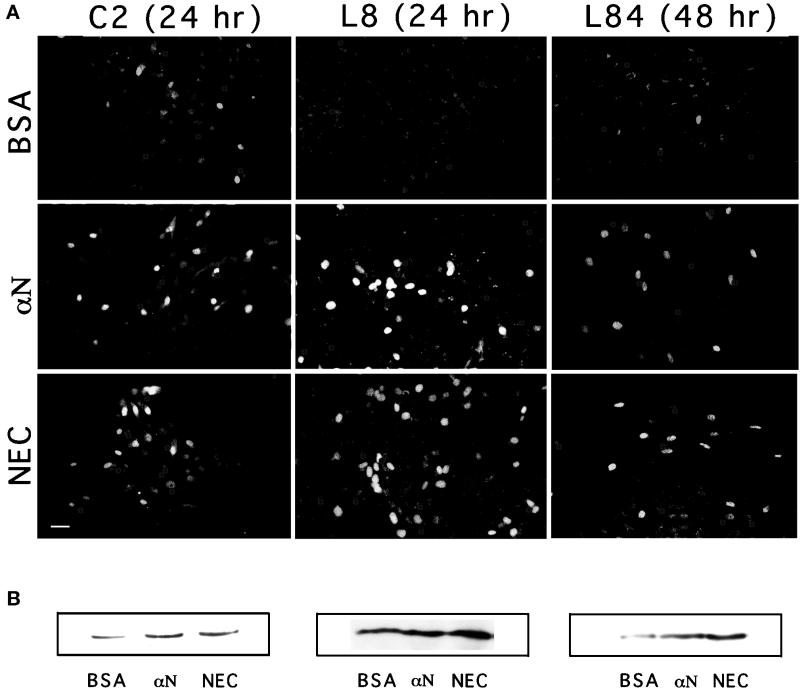
Figure 7.
Effects of N-cadherin stimulation on myogenin expression in cultured myoblasts. C2, L8, or L84 myoblasts were seeded and incubated overnight on gelatin-coated coverslips, treated with different beads, as indicated, permeabilized, fixed, and immunostained with anti-myogenin antibodies (A). The number of cells, as determined by DAPI staining, was approximately equal in all fields, and the percentage of bead-associated cells was ∼25%. Notice the overall increase in the number of myogenin-positive nuclei in cells after treatment with the cadherin-reactive beads. Bar, 20 μm. (B) Immunoblot analysis of total cell extracts prepared from the three myogenic lines, treated as in A. αN, anti-N-cadherin antibodies.
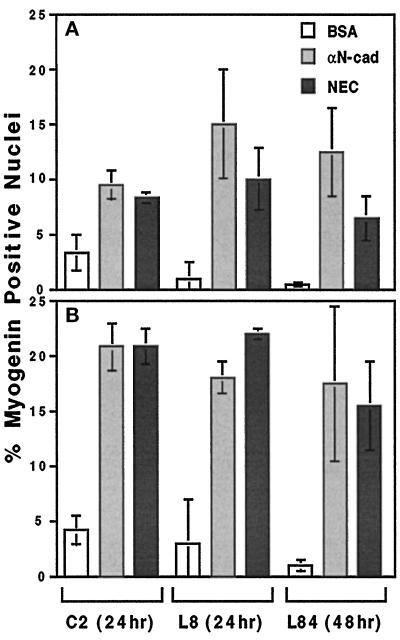
Figure 8.
Effect of cadherin-reactive beads on the number of myogenin-positive cells in C2, L8, and L84 myoblast cultures. Cells were plated and treated with different beads as described in Figure 7. The percentage of myogenin positive nuclei in the entire culture (A) or in the subpopulation of bead-associated cells (B) was calculated. Each column represents the mean ± SD of 3 independent experiments, each in duplicate, counting a total of 1000 cells in every case in A and 250 cells in each case in B. αN-cad, anti-N-cadherin antibodies.
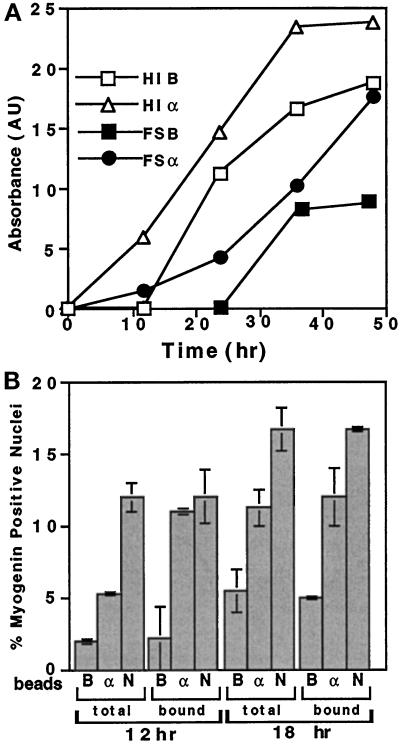
Figure 9.
Effect of cadherin-reactive beads on myogenin expression in sparse cultures of C2 myoblasts. C2 cells were seeded and cultured overnight at 10% confluence, and then the medium was replaced with fresh FCS-containing “growth medium” (FS) or with “differentiation medium” containing horse serum and insulin (HI), and the various beads were added. (A) Total cell extracts were prepared at the indicated time points after addition of beads, subjected to SDS-PAGE, transferred to nitrocellulose sheets, and reacted with anti-myogenin antibodies. The absorbance of the myogenin bands, determined by densitometry, is shown. (B) The percentage of the myogenin positive nuclei after treatment was calculated. The cells were maintained in growth medium and treated with beads for 12 or 18 h, fixed, and immunolabeled for myogenin. The percentage of positive cells was calculated out of the entire population or the subpopulation of bead-associated cells. Every column represents the mean ± SD of 3 independent experiments, each in duplicate. In each individual experiment a total number of 900 cells or 200 bead-bound cells were counted. B, BSA; α, anti-N-cadherin antibodies; N, NEC.
The expression of myogenin was also elevated in cultures of sparsely plated C2 cells, which rarely interact with neighboring cells, and do not readily fuse into myotubes. As shown in Figure 9, when such C2 cells are treated with beads for different periods and under different growth conditions, a significant increase in the myogenin level (Figure 9A) and in the number of myogenin-positive cells (Figure 9B) is detected among the cadherin-stimulated myoblasts compared with the BSA controls. Myogenin-positive nuclei are first observed 12 h after addition of beads to the sparsely plated C2 myoblasts cultured in growth medium.
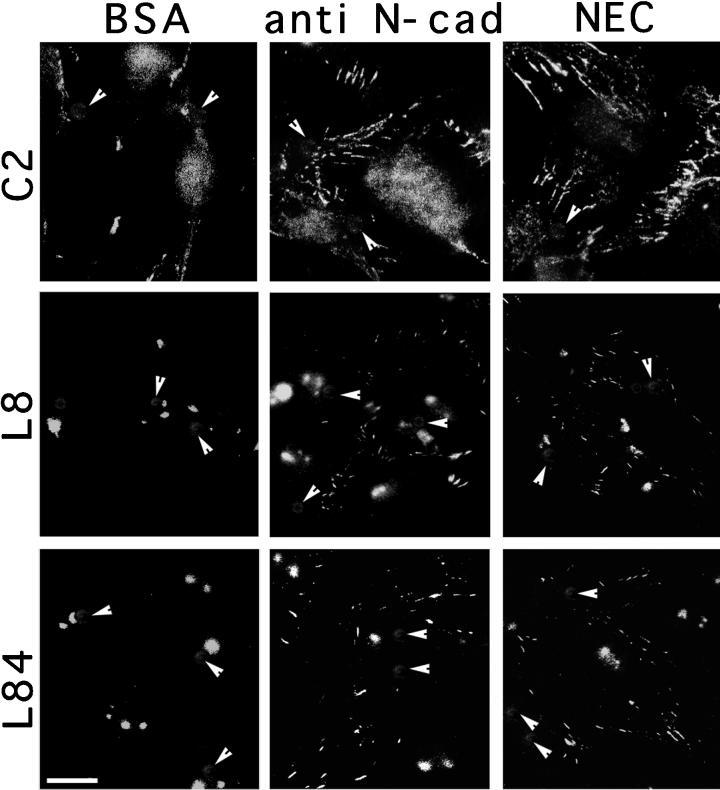
Enhancement of Cell–Cell Adhesion by Cadherin-reactive Beads
To further elucidate the mechanism responsible for N-cadherin–mediated myogenic differentiation, we have examined the effect of the various beads on cell–cell interactions. It was recently demonstrated that clustering of N-cadherin by bead-associated cadherin ligands specifically enhances cell–cell adherens junction formation (Levenberg et al., 1998a). We have thus examined the distribution of cadherin and β-catenin in cells treated with NEC- or BE-coated beads. As shown in Figure 10, treatment with the cadherin ligands elevates β-catenin labeling at cell–cell junctions within a few hours after addition of beads. Phase-contrast microscopy indicated that this increase in adherens junction formation is also accompanied by a general assembly of cells into more coherent arrays (our unpublished results). However, no significant changes in the overall levels of β-catenin or cadherin were noted (our unpublished results).
Figure 10.
Effect of cadherin stimulation on adherens junction formation in myogenic cell lines. C2, L8, and L84 cells were sparsely plated and further incubated for 10 h on gelatin-coated coverslips. The various beads were added to the cells for 6 h in growth medium. The cells were then permeabilized, fixed, and immunostained for β-catenin. Notice the intensive staining of the cell–cell contact sites and the apparent increase in staining after treatment with the cadherin-reactive beads. The positions of beads are indicated by arrowheads. Bar, 10 μm. anti N-cad, anti-N-cadherin antibodies.
DISCUSSION
Cadherins are important morphoregulatory molecules that are involved in homophilic adhesion of cells. N-cadherin is a member of the cadherin superfamily, which plays a crucial role in embryonic morphogenesis, including muscle development. Previous studies indicated that stable adhesive interactions must be established between fusion-competent myoblasts, as a prerequisite for further differentiation, and that these initial adhesions are calcium dependent (Knudsen et al., 1990). Specific involvement of N-cadherin was also suggested on the basis of its high levels in prefusion myoblasts (MacCalman et al., 1992). Moreover, the perturbation of N-cadherin–mediated adhesion in vitro affected the rate (but not the final level) of myoblast fusion (Mege et al., 1992). It was suggested by other studies that N-cadherin may not be essential for myotube formation, because specific blocking of M-cadherin (a muscle-specific form) by inhibitory antibodies blocks the fusion of cultured L6 myocytes (Zeschingk et al., 1995). In addition, myoblasts from N-cadherin–null mice are able to fuse both in culture and in vivo (Charlton et al., 1997). In view of the complexity of the various systems discussed above, we have attempted, in the present study, to examine the direct involvement of N-cadherin in myogenesis by its clustering with specific ligands, namely, NEC or anti-N-cadherin antibodies.
Here we present evidence that local clustering or immobilization of N-cadherin triggers signaling events that activate the myogenic program in several cultured myoblast lines. We found that the cadherin-reactive beads activate and facilitate the myogenic program, including myotube formation, expression of a variety of sarcomeric components, and expression of the myogenic transcription factor myogenin. Interestingly, myotube formation depends on high cell density even after cadherin bead stimulation, whereas the expression of the different muscle proteins, including myogenin, was also detected in sparse cultures, apparently independently of cell fusion. This is in line with the common sequence of myogenic events triggered by growth factor withdrawal, which start with myogenin expression, induction of growth arrest, expression of structural sarcomeric components, and, finally, fusion into myotubes (Andrés and Walsh, 1996). It is, however, noteworthy that the growth arrest induced by N-cadherin–reactive beads is not unique to the myogenic differentiation pathway, and treatment of a variety of mesenchymal cells with the same types of beads inhibits proliferation and blocks the cell cycle at the G1 phase. The mechanism underlying this growth inhibiting signaling process will be described in detail elsewhere (Levenberg et al., 1998b). Our data are consistent with the notion that growth arrest precedes the expression of the various structural sarcomeric components by ∼24 h.
The crucial events in skeletal muscle differentiation are coordinated by the expression of muscle regulatory proteins that act in cooperation with the MEF2 family of transcription factors to activate muscle-specific gene expression (Yun and Wold, 1996). These proteins were also shown to interact with and be regulated by other transcription factors and the cell cycle regulatory system to coordinately activate the differentiation program and to inhibit proliferation (Olson, 1992, 1993; Rao et al., 1994; Skapek et al., 1995, 1996). The fine balance between proliferation and differentiation appears to be critical for the induction and progression of the myogenic program. For instance, in committed myoblasts MyoD and Myf5 proteins are expressed, although their activity is apparently inhibited by the presence of growth-promoting factors, and thus the progression of differentiation depends on growth factor withdrawal, leading to myogenin expression and activation of the myogenic cascade (Andrés and Walsh, 1996).
Numerous studies indicate that in the course of myogenic differentiation inhibition of cell proliferation and cell death are coordinately regulated, and the inability to exit the cell cycle leads to apoptotic death (Walsh and Perlman, 1997; Fimia et al., 1998). Cell cycle inhibitors, such as p21 or Rb, are able to prevent this apoptosis most probably by the induction of cell cycle arrest (Wang and Walsh, 1996; Wang et al., 1997; Zacksenhaus et al., 1996). As described above, treatment with cadherin-reactive beads inhibits cell cycle progression in C2 myoblasts. However, no apparent differences in the number of apoptotic nuclei (defined by DAPI staining) were observed after application of the various beads (our unpublished results). Current reports demonstrate that the decision to exit the cell cycle and further differentiate or to die is made at the level of myogenin-induced cell cycle arrest, i.e., at the stages of myogenesis when cells already express myogenin. Because cadherin-reactive beads promote myogenin expression, it seems to us unlikely that stimulation of cadherin-mediated adhesion directly affects the apoptotic process.
Another aspect raised by the present study is the specificity of the effects on myogenesis to N-cadherin. As indicated above, additional members of the cadherin family are also expressed in muscle tissues, including M- and R-cadherins (Zeschingk et al., 1995; Rosenberg et al., 1997) and cadherin-11 (Kimura et al., 1995), and perturbation of some of these can affect myogenesis (Zeschingk et al., 1995). We have no direct evidence or claim that the effect shown here for N-cadherin stimulation is unique to this isoform and cannot be obtained by the clustering or immobilization of other cadherins. It is noteworthy that these three cadherins show considerable overall homology with N-cadherin along their cytoplasmic domains (82, 50, and 54% identity), which are presumably involved in the transduction of N-cadherin–mediated signals.
The data presented here are in agreement with the view that activation of cadherin-mediated signaling leads to the expression of myogenin, which in turn inhibits cell cycle progression, triggers the differentiation program, including the expression of sarcomeric proteins, and promotes myotube formation. The mechanism underlying this cadherin-induced activation of myogenin expression is, however, not clear. It was previously shown that cadherin-reactive beads specifically activate tyrosine phosphorylation at adherens junctions and enhance cadherin-mediated cell–cell adhesion in a variety of mesenchymal cells (Levenberg et al., 1998). This is consistent with the present results, showing that cadherin-induced stimulation leads to a specific and generalized enhancement of myoblast–myoblast adhesion (Figure 10). This, in turn, could have two distinct effects that are highly relevant to the progression of myogenic differentiation: 1) the signals triggered by the beads might be directly involved in the stimulation of myogenin expression; and 2) the apparent increase in cell adhesion, triggered by the beads, might further promote the myogenin-induced progression of differentiation.
Another possible pathway for cadherin-induced effects might involve the catenin system. β-Catenin, which is an intrinsic component of adherens junctions, is also implicated in Wnt and Wg signaling (Willert and Nusse, 1998) and in malignant transformation (Korinek et al., 1997; Morin et al., 1997; Redfield et al., 1997). In view of the capacity of extrajunctional β-catenin to translocate to the nucleus and to be involved in gene transactivation, together with LEF and Tcf transcription factors (Cavallo et al., 1997), it might be interesting to explore the possibility that some of the genes whose expression is regulated during myogenesis are under the control of β-catenin, and that changes in β-catenin stability, localization, and/or activity might affect the myogenic process. Some of these aspects are currently under study.
ACKNOWLEDGMENTS
We express our gratitude to Dr. D. Yaffe (The Weizmann Institute) for many illuminating discussions and helpful advice, as well as for providing the various cell lines used in this study. We are grateful to Ilana Sabanay for her expert help with the electron microscopic work and to Drs. B. Winter, H.H. Arnold, W. Obermann, D. Fürst, and M. Wheelock for providing antibodies used in this study. This work was supported by the Israel Research Foundation and by the Rita Markus Foundation. B.G. holds the Erwin Neter Chair in Cell and Tumor Biology.
REFERENCES
- Andrés V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon O, Geiger B. Cyclic changes in the organization of cell adhesions and the associated cytoskeleton, induced by stimulation of tyrosine phosphorylation on bovine aortic endothelial cells. J Cell Sci. 1997;110:547–556. doi: 10.1242/jcs.110.5.547. [DOI] [PubMed] [Google Scholar]
- Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. Bioessays. 1995;17:97–99. doi: 10.1002/bies.950170203. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherins expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Cavallo R, Rubinstein D, Peifer M. Armadillo and dTCF: a marriage made in the nucleus. Curr Opin Genet Dev. 1997;7:459–466. doi: 10.1016/s0959-437x(97)80071-8. [DOI] [PubMed] [Google Scholar]
- Charlton CA, Mohler WA, Radice GL, Hynes RO, Blau HM. Fusion competence of myoblasts rendered genetically null for N-cadherin in culture. J Cell Biol. 1997;138:331–336. doi: 10.1083/jcb.138.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Walsh FS. Signal transduction events underlying neurite outgrowth stimulated by cell adhesion molecules. Curr Opin Neurobiol. 1994;4:49–95. doi: 10.1016/0959-4388(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Fimia GM, Gottifredi V, Belli B, Ricciardi MR, Tafuri A, Amati P, Maione R. The activity of differentiation factors induced apoptosis in polyoma virus large T-expressing myoblasts. Mol Biol Cell. 1998;9:1449–1463. doi: 10.1091/mbc.9.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Yehuda-Levenberg S. Molecular interactions in the submembrane plaque of cell-cell and cell-matrix adhesions. Acta Anat. 1995;154:46–62. doi: 10.1159/000147751. [DOI] [PubMed] [Google Scholar]
- Geiger B, Ginsberg D, Salomon D, Volberg T. The molecular basis for the assembly and modulation of adherens-type junctions. Cell Differ Dev. 1990;32:343–53. doi: 10.1016/0922-3371(90)90049-3. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Gehart J, Blitz J, Simak E, Knudsen KA. N-cadherin promotes the commitment and differentiation of skeletal muscle precursor cells. Dev Biol. 1997;185:14–24. doi: 10.1006/dbio.1997.8542. [DOI] [PubMed] [Google Scholar]
- Holt CE, Lemaire P, Gurdon JB. Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopus mesoderm. Proc Natl Acad Sci USA. 1994;91:10844–10848. doi: 10.1073/pnas.91.23.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Biol Dev. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- Knudsen KA. Cell adhesion molecules in myogenesis. Curr Opin Cell Biol. 1990;2:902–906. doi: 10.1016/0955-0674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Myers L, McElwee S. A role for the Ca2+-dependent adhesion molecules, N-cadherin, in myoblast interaction during myogenesis. Exp Cell Res. 1990;188:175–184. doi: 10.1016/0014-4827(90)90157-6. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complexes in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Katz B-Z, Yamada K, Geiger B. Long-range and selective autoregulation of cell-cell or cell-matrix adhesions by cadherin or integrin ligands. J Cell Sci. 1998a;111:347–357. doi: 10.1242/jcs.111.3.347. [DOI] [PubMed] [Google Scholar]
- Levenberg, S., Yarden, A., Kam, Z., and Geiger, B. (1998b). p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene (in press). [DOI] [PubMed]
- MacCalman C, Bardeesy N, Holland PC, Blaschuk OW. Noncoordinated developmental regulation of N-cadherin, N-CAM, integrin, and fibronectin mRNA levels during myoblast terminal differentiation. Dev Dyn. 1992;195:127–132. doi: 10.1002/aja.1001950207. [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Chen H, Boyce BF, Mundy GR, Yoneda T. The role of cadherin in the generation of multinucleated osteoclasts from mononucleated precursors in murine marrow. J Clin Invest. 1995;95:2757–2765. doi: 10.1172/JCI117979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege RM, Goudou D, Diaz C, Nicolet M, Garcia L, Geraud G, Rieger F. N-cadherin and N-CAM in myoblast fusion: compared localization and effect of blockage by peptides and antibodies. J Cell Sci. 1992;103:897–906. doi: 10.1242/jcs.103.4.897. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks A, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:11787–11789. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154:261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol. 1993;7:1369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- Olson EN, Klein WH. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;8:1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995;267:386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Rao SS, Chu C, Stave Kontz D. Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix-loop-helix regulators. Mol Cell Biol. 1994;14:5259–5267. doi: 10.1128/mcb.14.8.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield A, Nieman MT, Knudsen KA. Cadherins promote skeletal muscle differentiation in three-dimensional cultures. J Cell Biol. 1997;138:1323–1331. doi: 10.1083/jcb.138.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues Fernandez JL, Geiger B, Salomon D, Ben Ze’ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility, and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993;122:1285–1294. doi: 10.1083/jcb.122.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P, Esni F, Sjodin A, Larue L, Carlsson L, Gullberg D, Takeichi M, Kemler R, Semb H. A potential role of R-cadherin in striated muscle formation. Dev Biol. 1997;187:55–70. doi: 10.1006/dbio.1997.8602. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehman MS, Grubel G, Legrand J-F, Als-Neilsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–333. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Kim PS, Novitch BG, Lassar AB. Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRb hyperphosphorylation. Mol Cell Biol. 1996;16:7043–7053. doi: 10.1128/mcb.16.12.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Itoh M, Nagafuchi A, Yonamura S, Tsukita S. Submembranous junctional plaque proteins include potential tumor suppressor molecules. J Cell Biol. 1993;123:1049–1053. doi: 10.1083/jcb.123.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Kam Z, Pankov R, Simcha I, Sabanay H, Coll JL, Adamson E, Ben-Ze’ev A. Focal adhesion formation by F9 embryonal carcinoma cells after vinculin gene disruption. J Cell Sci. 1995;108:2253–2260. doi: 10.1242/jcs.108.6.2253. [DOI] [PubMed] [Google Scholar]
- Volberg T, Zick Y, Dror R, Sabanay I, Gilon C, Levitzki A, Geiger B. The effect of tyrosine-specific protein phosphorylation on the assembly of adherens-type junctions. EMBO J. 1992;11:1733–1742. doi: 10.1002/j.1460-2075.1992.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. II.Antibody-mediated modulation of junction formation. J Cell Biol. 1986;103:1451–1464. doi: 10.1083/jcb.103.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guo K, Wills KN, Walsh K. Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 1997;57:351–354. [PubMed] [Google Scholar]
- Wang J, Walsh K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- Willert K, Nusse R. β-catenin: a key modulator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1976;7:159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- Yun K, Wold B. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr Opin Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E, Jiang Z, Chung D, Marth JD, Phillips RA, Gallie BL. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev. 1996;10:3051–3064. doi: 10.1101/gad.10.23.3051. [DOI] [PubMed] [Google Scholar]
- Zeschingk M, Kozian D, Kuch C, Schamoll M, Starzinski-Powitz A. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J Cell Sci. 1995;108:2973–2981. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]