Abstract
Animal studies suggest that prostaglandins in skeletal muscles stimulate afferents and contribute to the exercise pressor reflex. However, human data regarding a role for prostaglandins in this reflex are varied; in part because of systemic effects of pharmacologic agents used to block prostaglandin synthesis. We hypothesized that local blockade of prostaglandin synthesis in exercising muscles could attenuate muscle sympathetic nerve activity (MSNA) responses to fatiguing exercise. Blood pressure (Finapres), heart rate, and MSNA (microneurography) were assessed in 12 young healthy subjects during static handgrip and post exercise muscle ischemia (PEMI) before and after local infusion of 6 mg ketorolac tromethamine in saline via Bier block (regional intravenous anesthesia). In the second experiment (n=10), the same amount of saline was infused via the Bier block. Ketorolac Bier block decreased the prostaglandins synthesis to ~33% of the baseline. After ketorolac Bier block, the increases in MSNA from the baseline during the fatiguing handgrip was significantly lower than that before the Bier block (before ketorolac: Δ502±111; post ketorolac: Δ348±62%, P=0.016). Moreover, the increase in total MSNA during PEMI after ketorolac was significantly lower than that before the Bier block (P=0.014). Saline Bier block had no similar effect. The observations indicate that blockade of prostaglandin synthesis attenuates MSNA responses seen during fatiguing handgrip, and suggest that prostaglandins contribute to the exercise pressor reflex.
Keywords: prostaglandins, exercise, nervous system, sympathetic, regional blood flow
INTRODUCTION
Exercise elicits increases in muscle sympathetic nerve activity (MSNA), peripheral vasoconstriction, heart rate, cardiac output, and blood pressure (27, 29). It is believed that inputs from mechanically and chemically sensitive afferents from the exercising muscles are primarily responsible for this exercise pressor reflex (19, 28, 29). Group III and IV afferent fibers in muscles are suggested to be involved in this reflex (21, 35). Kaufman and colleagues demonstrated that anesthetized cat triceps surae group III muscle afferents were predominantly mechanically sensitive, whereas unmyelinated group IV muscle afferents were chemically sensitive (15, 16).
A number of substances are potential muscle afferent stimulants (14). Metabolism of free arachidonic acid by cyclooxygenases (COX) and lipoxygenases leads to the formation of prostaglandins, thromboxanes, and leukotrienes. It is known that arachidonic acid stimulates group III mechano-sensitive afferent nerve fibers in the anesthetized cat (26). Moreover, prostaglandin levels rise during exercise in healthy humans (36). Animal studies showed that arachidonic acid and the metabolites of the COX (i.e. prostaglandins) stimulate muscle afferents and can alter the pressor response to muscle contraction (12, 25, 26, 32). Middlekauff et al. showed that COX inhibition with intrabrachial arterial indomethacin eliminated the reflex sympathetic activation during low levels of dynamic exercise (22). However, during vigorous or fatiguing exercise, the data for the role of prostaglandins in the exercise pressor reflex in humans are controversial (5, 6, 9). Fontana et al. (9) reported that intravenous infusion of ketoprofen, a COX inhibitor, attenuated the pressor response to isometric handgrip. However, other studies demonstrated that prostaglandins synthesis blockade by oral administration of COX inhibitors (e.g. indomethacin or ketoprofen) had no effect on the responses of blood pressure (5, 6) and MSNA (6) to exercise.
It should be noted that in these previous studies (5, 6, 9), that effects due to the systemic administration of COX inhibitors per se could not be excluded. For example, animal studies showed that administration of pharmacological substances into the isolated carotid sinus capable of reducing prostaglandins synthesis impairs both afferent baroreceptor and efferent baroreflex responses to baroreceptor activation and/or deactivation (1, 2). Therefore, the systemic effects of drug infusion could have a myriad of effects which could preclude a precise assessment of the specific intramuscular effects of prostaglandin inhibition. Moreover, varied approaches and study designs in these different studies may have contributed to the different cardiovascular effects noted. The roles of prostaglandins in evoking the exercise pressor reflex during vigorous and fatiguing exercise have not been examined under circumstances where prostaglandin production was modulated in a highly regionalized fashion. Bier block, a regional intravenous anesthesia technique, has been successfully used in infusion of vasoactive drugs (i.e. bretylium) into the exercising arm to change the local vascular conductance with less systemic effect (18). Therefore, the purpose of the present study was to examine the effects of locally altering prostaglandins in exercising muscle on sympathetic activation during fatiguing exercise. We hypothesized that local cyclooxygenase inhibition in exercising muscle would attenuate the MSNA to isometric handgrip exercise. COX inhibition in the exercising forearm was accomplished by local infusion of ketorolac via the Bier block technique.
METHODS
Subjects
Twelve subjects (8 male, 4 female, age: 25±1 (SE) yr; height: 177±2 cm, weight: 74±2 kg) participated in the study. All subjects were normotensive (supine blood pressures <140/90 mmHg), were not taking any medication, and were in good health. Subjects refrained from caffeine, alcohol, and exercise 24 hrs prior to the study. The experimental protocol was approved by the Institutional Review Board of the Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki. Each subject had the purposes and risks of the protocol explained to them before written informed consent was obtained.
Measurements
Blood pressure was recorded on a beat-by-beat basis from a finger via a Finapres device (Finapres, Ohmeda, Madison, WI). Resting blood pressures obtained from the Finapres were verified by an automated sphygmomanometer (Dinamap, Critikon, Tampa, FL). A standard electrocardiogram was used to monitor heart rate. Respiratory excursions were monitored with pneumography. Multifiber recordings of MSNA were obtained with a tungsten microelectrode inserted in the peroneal nerve of a leg. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The recording electrode was adjusted until a site was found in which muscle sympathetic bursts were clearly identified using previously established criteria (34). The nerve signal was amplified, a band-pass filtered with a bandwidth of 500–5000 Hz, and integrated with a time constant of 0.1 sec (Iowa Bioengineering, Iowa City, IA). The nerve signal was also routed to a loudspeaker and a computer for monitoring throughout the study. Heart rate, blood pressure, MSNA and respiratory excursions were recorded throughout the studies.
Brachial artery limb blood flow was determined by combined pulsed and echo Doppler ultrasound (ATL 5000, Philips Medical System, Bothell, WA) in 8 subjects. The Doppler shift signal was demodulated to provide instantaneous mean blood velocity data in real time. Vessel dimensions were measured at each workload by using echo Doppler sonography. Blood flow was determined from both the blood velocity and the vessel cross-sectional area. Venous samples were collected at the antecubital fossa of the exercising arm. Plasma thromboxane B2 was used to document the effectiveness of COX blockade (6, 9, 24). Thromboxane B2 levels were quantified by enzyme immunoassay (Amersham Biosciences).
Experimental Design
All subjects were tested in the supine position. An intravenous catheter was inserted in the antecubital fossa of the non-dominant arm. The maximal voluntary contraction (MVC) of the non-dominant hand was tested during each visit.
Control trial A
After the instrumentation, 6 min baseline data of heart rate, blood pressure, MSNA, respiratory excursion, and brachial blood flow were collected in the resting condition. A baseline blood sample was obtained. Then, progressive handgrip exercise was performed. Each subject performed static isometric handgrip at 15% MVC for 1 min and 25% MVC for 1 min. Then, the subject performed handgrip at 30% MVC to fatigue followed by 2.5 min of post exercise muscle ischemia (PEMI) via inflating a cuff on the upper arm to 250 mmHg. There were no rest periods between the workloads. During the initial period of low intensity handgrip, metaboreceptor engagement is not likely. At the end of the fatiguing exercise, mechanoreceptors, metaboreceptors and central command all may contribute to the exercise pressor responses. During PEMI, the MSNA and blood pressure responses should be only caused by the metaboreceptor inputs. Brachial blood flow was measured during the progressive handgrip exercise. One blood sample was drawn during PEMI.
Bier block
After 10–15 min of recovery from the control trial, the Bier block procedure was utilized to regionally administer ketorolac tromethamine, a non-selective COX inhibitor (3), to block prostaglandin synthesis in the forearm. In order to “drain” the forearm vasculature, the arm was elevated and bandaged with a tight elastic wrapping beginning at the hand. The pneumatic cuff on the upper arm was then inflated to 250 mmHg, and the bandage was removed. Thereafter, 6 mg ketorolac tromethamine in 40 ml of saline was infused into the occluded arm via the catheter (the dose of the drug was decided via pilot studies). This allows the ketorolac to distribute in the previously emptied vascular system and to diffuse into the forearm tissue. After 20 min, the cuff was deflated. The subjects rested for 15–20 min.
Ketorolac Bier block trial
Thereafter, another 6 min of baseline data was collected, and a blood sample was drawn. Then, progressive handgrip exercise at the same intensities as those employed prior to the Bier block trial followed by PEMI was repeated. One blood sample was drawn during PEMI.
To separate the effects of ketorolac from the Bier block procedure itself, a control study was performed in 10 subjects. This study was performed on a second visit to the laboratory a number of weeks after the first experiment. All parameters were recorded in the same fashion as the first visit. The Control trial B in the second visit was the same as the pre-ketorolac Control trial A performed in the first visit. During the Bier block procedure, 40 ml saline (no ketorolac) was infused into the arm. Then the progressive handgrip exercise and PEMI protocol was repeated for the saline Bier block trial.
Data Analysis
Data were sampled at 200 Hz via a data acquisition system (MacLab, AD Instruments, Castle Hill, Australia). Total MSNA was identified from burst area of the integrated neurogram (4). The total MSNA was also expressed in arbitrary units by assigning the mean burst area per minute value of 100 during the supine baseline before each trial. Mean arterial pressure (MAP) was calculated from the Finapres waveform during each stage of the progressive handgrip exercise and PEMI, while the baseline MAP was verified by an automated sphygmomanometer from an upper arm. Forearm vascular conductance (FVC) was calculated from the MAP and forearm blood flow. The final FVC was expressed in arbitrary units by assigning the rest baseline before each exercise trial a value of 100.
Statistics
Differences in the mean values of hemodynamic parameters between the baselines prior to the 4 exercise trials were evaluated via Tukey post-hoc analysis after repeated measures one-way ANOVA. Differences in the mean values of hemodynamic parameters between the baselines and exercise, and between the drug infusion conditions were evaluated via Tukey post-hoc analysis after repeated measures two-way ANOVA. All values are reported as means ± SE. P values of <0.05 were considered statistically significant.
RESULTS
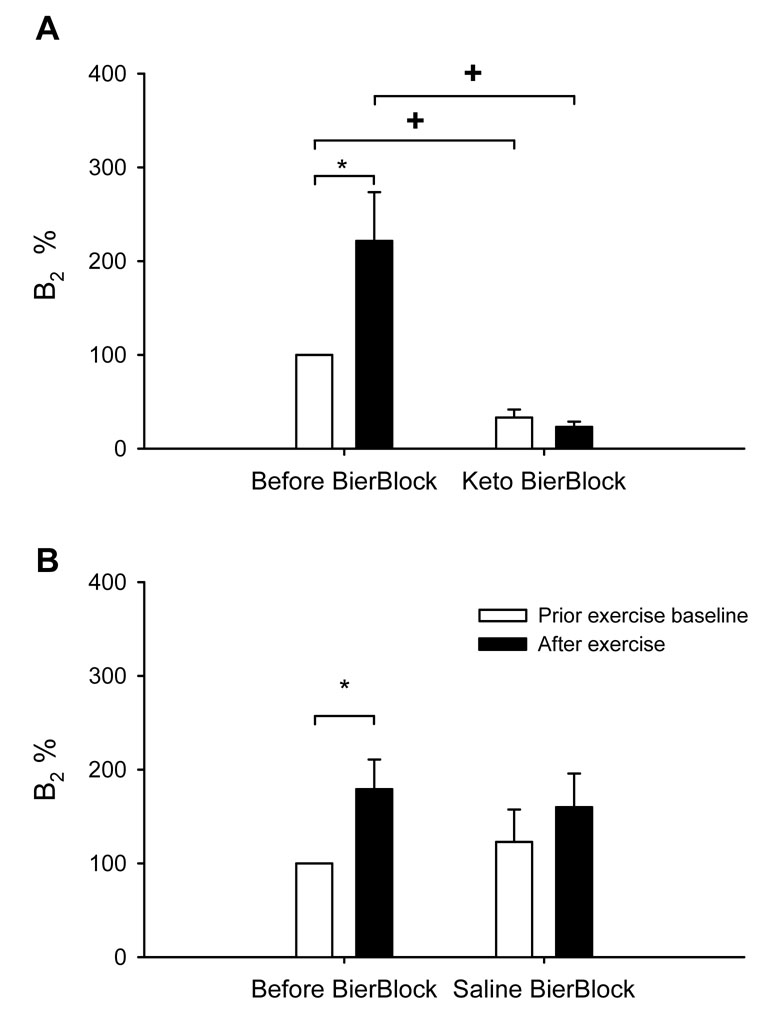
Baseline values for MSNA, MAP and heart rate obtained before the four trials did not differ (Table 1). Because both prostaglandins and thromboxane synthesis are COX dependent, the thromboxane B2 was used as a bioassay of COX antagonism (i.e. prostaglandin synthesis inhibition) in the present study (6, 9, 24). Resting plasma thromboxane B2 levels were significantly decreased after ketorolac Bier block (230±52 to 38±4 pg/ml, P<0.01). The effects of exercise on the thromboxane B2 levels are shown in Figure 1. Before the ketorolac Bier block thromboxane B2 rose with exercise; after the ketorolac Bier block, thromboxane B2 did not increase with exercise. In contrast, the saline Bier block had no similar effect on thromboxane B2 levels.
Table 1.
Prior Exercise Baseline Measurements.
| Before Ketorolac Bier Block | After Ketorolac Bier Block | Before Saline Bier Block | After Saline Bier Block | |
|---|---|---|---|---|
| SBP mmHg | 125±3 | 127±3 | 123±2 | 124±4 |
| DBP mmHg | 63±2 | 62±2 | 63±2 | 64±2 |
| MAP mmHg | 84±2 | 85±2 | 83±2 | 84±2 |
| Heart rate beats/min | 61±2 | 60±2 | 60±2 | 60±2 |
| MSNA bursts/min | 11.3±1.2 | 11.5±1.4 | 9.6±1.1 | 10.4±1.4 |
| MSNA units/min | 176±23 | 151±19 | 141±17 | 163±30 |
| Respiration cycles/min | 16.2±0.6 | 15.8±0.7 | 16.8±0.8 | 16.7±0.8 |
| Subject number | 12 | 12 | 10 | 10 |
Values are mean ± SE. SBP, DBP, MAP: systolic, diastolic and mean arterial blood pressure, which were measured by an automated sphygmomanometer from an upper arm. There is no significant difference in the measurements between the trials.
Figure 1.
Effects of ketorolac on thromboxane B2 levels. Panel A: before and after Keto Bier Block. Panel B: before and after saline Bier Block. The thromboxane B2 levels are expressed in arbitrary units by assigning the control baseline a value of 100. Subject number = 12. *: P<0.05 vs. the respective prior exercise baseline. †: P<0.05 vs. the respective control trial condition.
Effects of Ketorolac on Sympathetic Activation During Isometric Handgrip
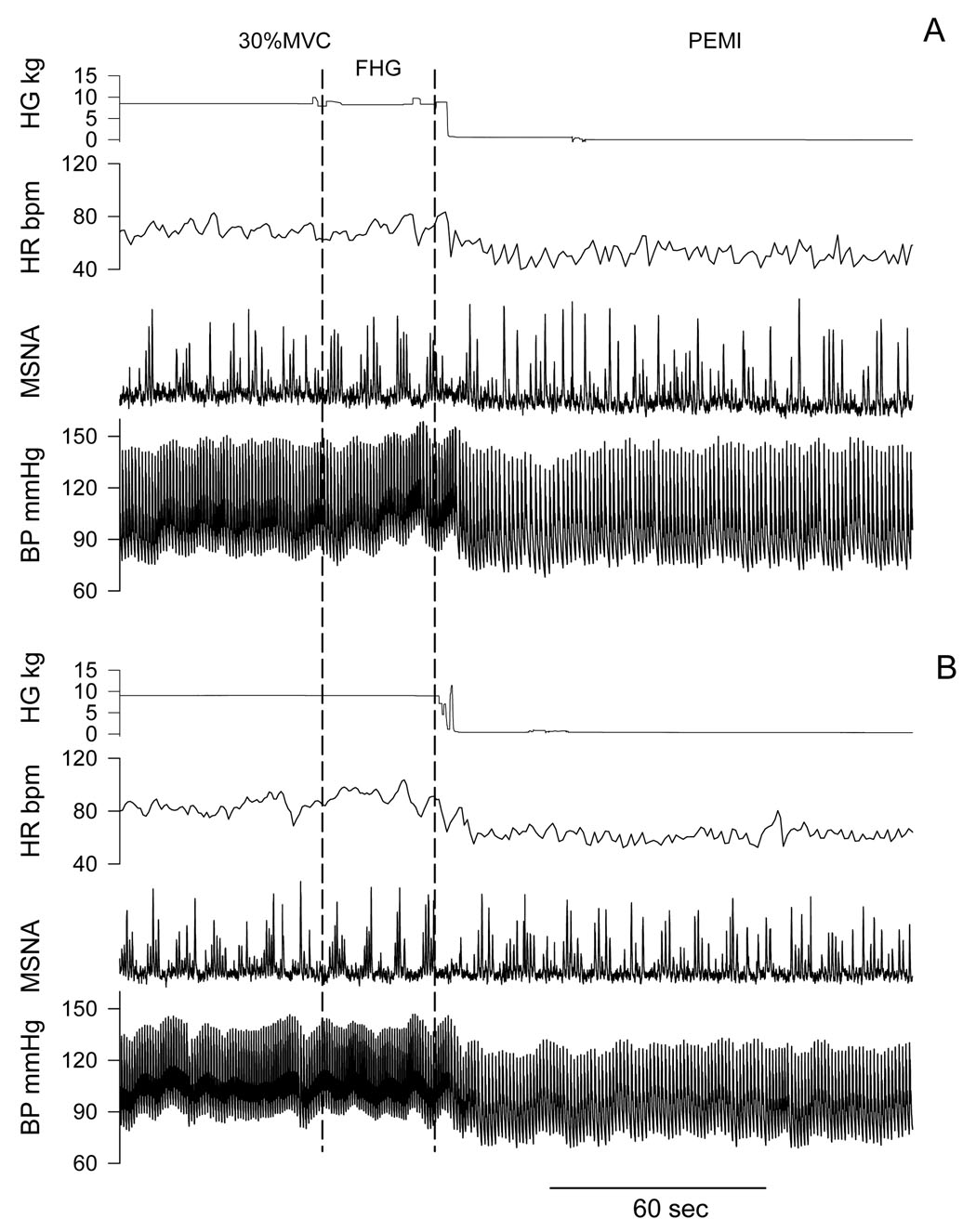
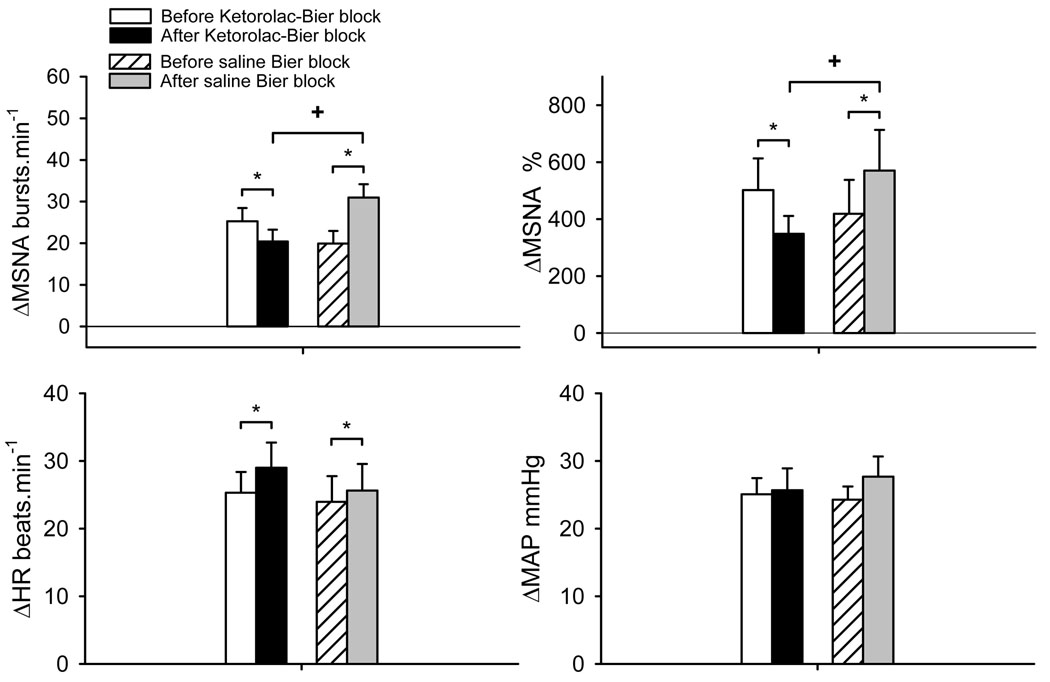
Recordings of handgrip force, heart rate, integrated MSNA and blood pressure before fatigue and PEMI in a representative subject are shown in Figure 2. Progressive handgrip evoked increases in MSNA, heart rate and MAP (Table 2). After the ketorolac Bier block, MSNA responses during the last 30 sec of handgrip before fatigue (FHG) were significantly lower than that before ketorolac Bier block (Figure 3). On the other hand, after the saline Bier block, MSNA responses during the first min of 30% MVC and FHG were significantly greater than that before saline Bier block. Thus, after the ketorolac Bier block, the MSNA responses at these stages were significantly lower than those after saline Bier block.
Figure 2.
Representative tracing of handgrip force, heart rate, muscle sympathetic nerve activity (MSNA) and arterial blood pressure (BP) during the last period of handgrip and post exercise ischemia (PEMI). Panel A: before Ketorolac Bier block. Panel B: after Ketorolac Bier block. FHG: the last 30 sec of handgrip before fatigue.
Table 2.
MSNA and Cardiovascular Responses During the Progressive Handgrip Exercise Before and After Ketorolac or Saline Bier Block.
| Before Ketorolac Bier Block | After Ketorolac Bier Block | Before Saline Bier Block | After Saline Bier Block | ||
|---|---|---|---|---|---|
| ΔMSNA | 15% MVC | −0.3±1.0 | −0.6±1.4 | −1.1±0.9 | 2.1±1.7 |
| bursts/min | 25% MVC | 5.1±2.4 | 5.7±3.1 | 3.4±2.4 | 8.1±2.5 |
| 30% MVC | 10.6±3.3* | 13.6±2.9*‡ | 10.0±3.2* | 21.6±3.8*† | |
| ΔMSNA | 15% MVC | −2.6±12.6 | −1.2±15.7 | −28.3±11.5 | 35.1±28.5 |
| % | 25% MVC | 55.3±30.2 | 78.3±36.9 | 52.7±37.0 | 107.1±53.7 |
| 30% MVC | 199.7±70.9* | 211.4±58.9*‡ | 167.2±69.8* | 393.6±126.9*† | |
| ΔHeart rate | 15% MVC | 8±2* | 7±2* | 10±3* | 8±2* |
| beats/min | 25% MVC | 11±2* | 14±2*† | 13±2* | 15±3* |
| 30% MVC | 17±3* | 23±3*† | 17±4* | 23±4*† | |
| ΔMAP | 15% MVC | 4±1 | 2±1 | 3±1 | 2±1 |
| mmHg | 25% MVC | 8±2* | 9±2* | 6±1* | 9±1* |
| 30% MVC | 14±2* | 17±3* | 13±2* | 28±3*† |
The values are changes from the prior exercise baseline. 15% MVC, 25% MVC: the responses during 15% MVC (1 min) and 25% MVC (1 min) handgrip. 30% MVC: the responses during the first min of 30% MVC handgrip. ΔMSNA% shows the total activity of MSNA response.
P<0.05 vs. respective rest baseline.
P<0.05 vs. respective control trial.
P<0.05 vs. saline Bier block trial.
Figure 3.
MSNA and cardiovascular responses during the fatiguing handgrip exercise before and after local administration of ketorolac or saline into the exercising arm via Bier block procedure. The values are changes from the prior exercise baseline. The responses were during the last 30 sec handgrip before fatigue (see Figure 2). ΔMSNA% shows the total activity of MSNA response. The MSNA, heart rate (HR) and MAP during the fatiguing handgrip in all 4 trials were significantly greater than the rest baselines (all P<0.001). *: P<0.05 vs. respective control trial. †: P<0.05 vs. Ketorolac Bier block trial.
Heart rate increased significantly during all stages of the progressive handgrip exercise in all conditions (all P<0.05). After Bier block, the heart rate responses were greater than those in the respective control trial at 30% MVC and FHG. However, there was no significant difference between the ketorolac Bier block and saline Bier block conditions. After ketorolac Bier block, the MAP response during FHG was not significantly different from that before ketorolac Bier block.
After the ketorolac Bier block, the handgrip time to fatigue (including the first 2 minutes) tended to be shorter than that prior to the Bier block (231±17 vs. 277±13 sec, P=0.07). After the saline Bier block, the handgrip time to fatigue was significantly shorter than that prior Bier block (208±7 vs. 274±20 sec, P<0.001). The handgrip time after ketorolac Bier block was not significantly different from that after saline Bier block (P=0.23).
Effects of Ketorolac During PEMI
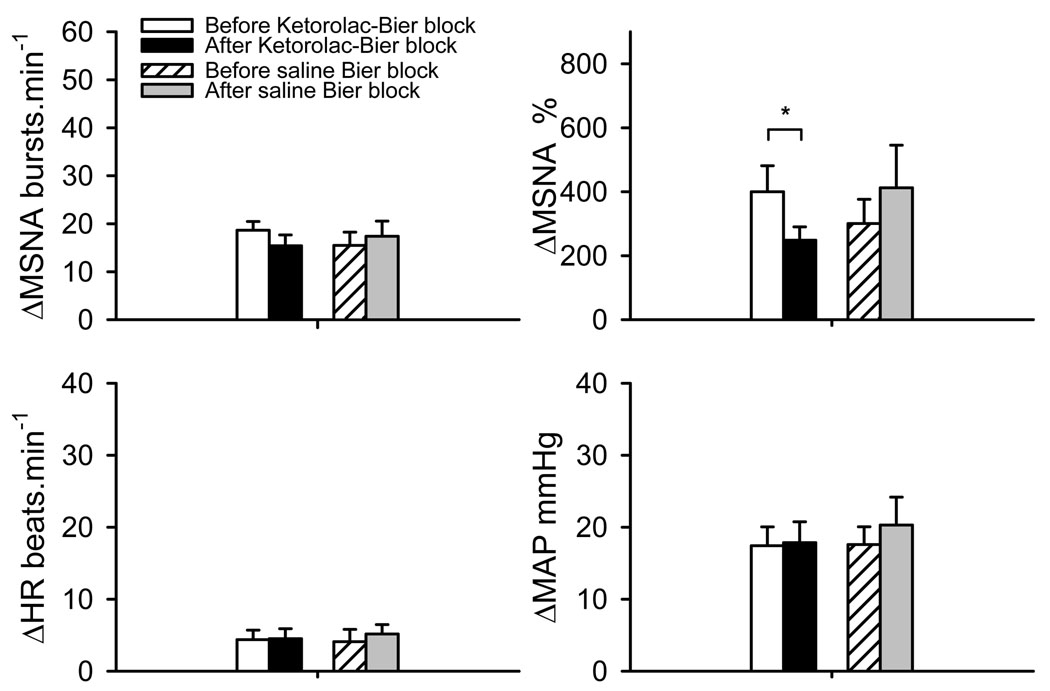
MSNA and MAP during PEMI in all of the 4 trials were significantly greater than the prior exercise baseline (Figure 4, all P<0.001). When MSNA was expressed as burst rate change from prior exercise baseline, MSNA during PEMI after ketorolac Bier block tended to be lower than that before the ketorolac Bier block (P=0.08). Moreover, the total activity of MSNA during PEMI after ketorolac Bier block was significantly lower than that before the ketorolac Bier block (P=0.014). There was no significant differences in MAP and heart rate responses to PEMI between the trials.
Figure 4.
MSNA and cardiovascular response to post exercise muscle ischemia (PEMI) after fatiguing handgrip before and after local administration of ketorolac or saline into the exercising arm via Bier block procedure. The data over the last 2 min of 2.5 min PEMI were analyzed. The values are changes from the prior exercise baseline. ΔMSNA% shows the total activity of MSNA response. The MSNA and MAP during the PEMI in all 4 trials were significantly greater than the rest baselines (all P<0.001). *: P<0.05 vs. respective control trial.
FVC Response
FVC of the exercising arm increased significantly from baseline during the handgrip exercise in all conditions (Table 3). After ketorolac Bier block, the FVC response at the first min of 30% MVC and FHG were significantly lower than those before ketorolac Bier block. This result indicates that the local vasodilation in the exercising muscles is attenuated after ketorolac administration. In contrast, the saline Bier block had no similar effect.
Table 3.
Forearm Vascular Conductance Responses During the Progressive Handgrip Exercise Before and After Ketorolac or Saline Bier Block.
| Exercise Level | Before Ketorolac Bier Block | After Ketorolac Bier Block | Before Saline Bier Block | After Saline Bier Block |
|---|---|---|---|---|
| 15% MVC | 197±40* | 132±29* | 161±29* | 179±63* |
| 25% MVC | 228±45* | 137±30* | 182±31* | 199±50* |
| 30% MVC | 320±66* | 165±37*† | 233±15* | 217±69* |
| FHG | 284±59* | 147±35*† | 198±12* | 192±64* |
The values are the percent change from the prior exercise baseline. FHG: the responses during the last 30 sec before fatigue. Subject number = 8.
P<0.05 vs. prior exercise baseline.
P <0.05 vs. before ketorolac Bier block.
DISCUSSION
The main findings from the present study are that local administration of a COX antagonist in the exercising muscles, which suppresses endogenous prostaglandins synthesis, attenuates the MSNA responses to fatiguing handgrip. Moreover, the MSNA responses to PEMI are also attenuated by the blockade of prostaglandins synthesis. These results verified our hypothesis and suggest that prostaglandins contribute to the sympathetic activation to fatiguing exercise.
Effect of Local Administration of Ketorolac
COX plays a critical role in transformation of free arachidonic acid to prostaglandins and thromboxanes (30, 31). Processes such as pharmacologically antagonizing COX with a nonsteroidal anti-inflammatory drug (NSAID) are an effective strategy to inhibit prostaglandins synthesis in vivo (11). Ketorolac is a powerful NSAID available for intravenous administration that antagonizes COX (3). Because both prostaglandins and thromboxane synthesis are COX dependent, the thromboxanes (i.e. thromboxane B2) were used as a bioassay of COX antagonism (i.e. prostaglandin synthesis inhibition). In the present study, the significant increase in thromboxane B2 level after exercise during the control conditions indicated an increase of prostaglandins, which is consistent with prior observations (36). After the ketorolac Bier block, the thromboxane B2 level decreased ~67 % from baseline. Moreover, there was no increase in thromboxane B2 level after the exercise in this condition. This result suggests that the prostaglandin synthesis was inhibited in the exercising muscles, and this effect was maintained during exercise. It should be noted that in a previous study, the thromboxane B2 was decreased ~53% by intravenous infusion of a large dose (45 mg) of ketorolac (24). In the present study, only 6 mg of ketorolac was locally infused into the exercising muscle with Bier block. Thus, local infusion of a small dose of ketorolac with the Bier block in the present study had a greater effect on prostaglandin synthesis inhibition than is seen when a much larger dose is given systemically. This result may also suggest that the infused drug could be mainly localized in the exercising arm. Thus, the local administration of a small dose of this drug should have far smaller systemic effects than those observed during intravenous and/or intra-arterial infusions. This point is supported by the observation that there were no differences in baseline MSNA, heart rate and blood pressure values seen during the 4 trials.
Responses to Handgrip
After the ketorolac Bier block, the MSNA response to fatiguing handgrip was significantly lower than that before ketorolac Bier block. Moreover, the MSNA response to fatiguing handgrip after saline Bier block was significantly greater than that before the ketorolac Bier block. These data indicate that MSNA response to fatiguing handgrip was attenuated by local infusion of ketorolac, and the decrease in MSNA response was not caused by the Bier block procedure itself. Actually, the 20 min ischemia in Bier block might accentuate MSNA responses. During the 20 min circulatory arrest, some metabolites might also accumulate in the arm tissues, which could accentuate the exercise pressor reflex after the Bier block. This could also cause the decrease in the exercise time after the Bier block. Alternately, the 40 ml saline in Bier block might slightly increase the volume of the forearm. A previous study showed that MSNA response to handgrip was accentuated when the forearm volume increased (20). Therefore, the present results demonstrate that the blockade of prostaglandin synthesis attenuated the MSNA response to the fatiguing exercise.
The present results are consistent with the observations from animal studies showing that arachidonic acid and prostaglandins can stimulate the muscle afferents and alter the pressor response to muscle contraction (25, 26, 32). Moreover, COX blockade attenuates the responses of group III and IV muscle afferents to dynamic exercise in cats (12). Middlekauff et al. (22) showed that the MSNA response to low level dynamic exercise was eliminated by COX inhibition via intra-arterial indomethacin. However, this study (22) only examined the role of prostaglandins during low level dynamic exercise. The present study was designed to examine the effect of prostaglandins production in exercising muscles on sympathetic and cardiovascular responses to fatiguing exercise. In the present study, trial differences in the MSNA responses during low level static handgrip were not seen. Cardiovascular responses during the initial period of low level exercise are small. Thus we speculate that the possible effects of ketorolac could not be separated from random variation in the cardiovascular variables. This is a limitation of static handgrip protocols performed at low intensities.
On the other hand, the results of previous human studies examining the effect of prostaglandins on cardiovascular responses to fatiguing exercise are equivocal. Davy et al. demonstrated that COX inhibition by oral administration of indomethacin had no effect on the arterial pressure response to isometric handgrip (5). In contrast, Fontana et al. (9) reported that intravenous infusion of ketoprofen, a COX inhibitor, attenuated the pressor response to isometric handgrip. However, Doerzbacher et al. showed that oral administration of ketoprofen had no effect on MSNA responses to isometric and dynamic handgrip exercise and PEMI (6). It should be emphasized that the varied results in these previous studies could be caused by the systemic effect of the drugs. For example, baroreceptor function might be altered by prostaglandin synthesis blockade (1, 2). Therefore, the systemic effects of drug infusion could have obscured the effects of local skeletal muscle prostaglandin synthesis inhibition on the autonomic responses to exercise. On the other hand, as discussed above, the local administration of a small dose of ketorolac in the present study had clear effects on the exercising muscles, while the systemic effects of the drug are likely to be smaller than the effects seen with the other delivery methods used in the prior reports. Thus, the result of the present study reveals the effects of local prostaglandins synthesis blockade in exercising muscles on the cardiovascular responses to exercise, and provides clear evidence that prostaglandins produced in the exercising muscles contribute to evoking sympathetic activation.
The heart rate responses in both Bier block trials were greater than those in the control trials. It was possible that central command influences after Bier block may be greater than central command input seen during the control trials. However, there was no significant difference between the ketorolac Bier block trial and the saline Bier block trial. This result is consistent with the observation of Middlekauff et al. (22). They reported that there was no significant difference in heart rate response to low level exercise between intra-arterial indomethacin and the saline trial, while the MSNA response was eliminated. Thus, it may be speculated that the prostaglandins in exercising muscle may play less of a role in mediating heart rate response. On the other hand, Fontana et al. (9) showed that intravenous administration of ketoprofen decreased heart rate responses to exercise. These differences could be caused by the differences in the drugs used, the dosages given and the method of administration employed. Moreover, it should be noted that maximal heart rate response evoked by 30% MVC in the present study was ~86 betas/min, while the maximal heart rate response seen with 40% MVC in the study of Fontana et al was near 120 beats/min. It is known that the changes in heart rate with low-intensity exercise are primarily mediated by vagal withdrawal, and that as exercise intensity increases, additional cardiovascular reactivity is mediated by increased sympathetic outflow (8, 13, 17). This could be one of the factors those caused the difference in the observations between the present report and the study of Fontana et al.
The MSNA response to handgrip after ketorolac Bier block was significantly lower than before ketorolac Bier block. However, there was no significant difference in MAP responses to the fatiguing exercise before and after ketorolac Bier block. As discussed above, the heart rate response might not be altered by the ketorolac infusion. Moreover, we speculate that the exercise induced sympathetic nerve responses directed to vascular beds other than muscle may not have been reduced by the ketorolac infusion (e.g. inner organs). The non-altered heart rate response and the postulated different responses in sympathetic outflow to other vascular beds might contribute to the lack of effect of ketorolac infusion on the pressor response to exercise.
Responses to PEMI
The present data show that total MSNA responses during PEMI after ketorolac Bier block were significantly lower than those seen before the ketorolac Bier block trial. This result may suggest that prostaglandins stimulate muscle afferents that contribute to the exercise pressor reflex. Rotto and colleagues showed that COX blockade attenuates response of group IV muscle afferents in cats (25). The results of Hayes et al. also suggested that COX products play a role in stimulating group IV afferents during post-exercise circulatory occlusion (12). The group IV muscle afferents are suggested to be chemically sensitive (15, 16). The present result is different from the observation of Doerzbacher et al. (6), which showed no difference in MSNA and MAP responses to PEMI between blockade of prostaglandins synthesis via oral ketoprofen and the placebo trial. The difference in the observations can be caused by the difference in drug administration methods.
Although the total MSNA response to PEMI after ketorolac Bier block was significantly lower than before the Bier block trial, it is interesting to note that there was no significant difference (P=0.08) in the MSNA burst rate during PEMI between the 2 trials. These data may indicate that the difference in MSNA response to PEMI between the 2 trials was not very large, since the difference could be only detected with a more sensitive method (total activity). On the other hand, during FHG, both the burst rate and the total activity of MSNA after ketorolac Bier block were significantly lower than those before the ketorolac Bier block trial. These observations may indicate that the difference in MSNA responses between the 2 trials during FHG was greater than that during PEMI. The most likely explanation for this observation is that prostaglandins may also sensitize the mechanoreceptors in muscles. Rotto et al. (26) have shown that COX blockade by indomethacin significantly inhibited sympathetic activation during rhythmic muscle contraction in anesthetized cats, a finding consistent with the concept that group III mechanosensory neurons are sensitized to mechanical stimuli by COX metabolites. In humans, Middlekauff et al. (22) demonstrated that COX inhibition with indomethacin eliminated MSNA response during low levels of dynamic exercise, and they concluded that COX products sensitize the muscle mechanoreceptors. The present results may hint that prostaglandins can also sensitize mechanoreceptors during fatiguing handgrip. This perspective should be verified in future studies.
FVC Response
It is well known that exercise evokes skeletal muscle vasodilation. It has been suggested that metabolites of arachidonic acid play an important role in the regulation of vascular tone (10). Compared with the control trial, the increase of FVC after blockade of prostaglandins was lower in the present study. This observation is consistent with a previous study (36). It should be noted that this attenuated vasodilatory response was not caused by the change in sympathetic activation, since MSNA response was attenuated in this condition. Thus, the attenuated vasodilation was a local effect of prostaglandin synthesis blockade. Therefore, the present and previous (36) results suggest that local synthesis and release of prostaglandins contribute to the local vasodilation in exercising muscles. It should also be noted that this attenuated vasodilatory response might not be caused by reduced thromboxane B2, since it is a metabolite of the potent vasoconstrictor thromboxane A2. Because the ketorolac tromethamine is a non-selective COX inhibitor (3), infusion of ketorolac would block/reduce the synthesis of both vasodilator prostaglandins and potent vasoconstrictor thromboxane. The measured blood flow responses reflect the cumulative effects of these various metabolites of arachidonic acid. Thus, the attenuated blood flow response to exercise would suggest that the primary effect of the ketorolac was to reduce vasodilator prostaglandin synthesis.
Based on the present results, we speculate that prostaglandins contribute to the exercise pressor reflex and local exercise induced vasodilation in healthy humans. As such, they may contribute to blood flow distribution during exercise. This role of the prostaglandins in diseases, such as heart failure, may be even more important. It has been reported that the plasma levels of prostaglandin metabolites are 3–10 times higher in patients with heart failure than in normal subjects, and the circulating levels of the prostaglandin metabolites are correlated with increased levels of vasoconstrictor hormones in the patients (7). On the other hand, the muscle mechanoreceptor activity may be enhanced (23) and muscle metaboreceptor activity may be attenuated in the patients (33). Thus, the potential relationship between the increased prostaglandin levels and the altered exercise pressor reflex in the heart failure subjects should be investigated in further studies.
Study Limitations
Because the half-life of ketorolac is ~7.6 hours, the ketorolac Bier block trial was always performed after the control trial. Thus, we cannot exclude some order effects. Moreover, the Bier block procedure itself could influence the observed responses. To separate these factors, the saline Bier block trial was performed. This study design decreased the influences of trial order and the Bier block procedure per se.
In conclusion, the present results show that local blockade of prostaglandins synthesis in exercising muscle attenuates the MSNA responses to fatiguing static handgrip exercise. Moreover, the MSNA responses seen during PEMI are also attenuated. These observations suggest that prostaglandins stimulate muscle afferent nerves during exercise and contribute to sympathetic activation seen during fatiguing handgrip. We postulate that this effect is due to both stimulation of metabolite sensitive afferents as well as to the sensitization of mechanically sensitive muscle afferents.
ACKNOWLEDGEMENTS
We are grateful to Jennifer L. Stoner for secretarial help in preparing this manuscript and Stephen F. Gugoff for technical support.
GRANTS
This work was supported by National Institutes of Health Grants P01 HL077670 (Sinoway), NIH/NCRR grants M01 RR010732 (GCRC Grant) and C06 RR016499 (Construction Grant), and the American Heart Association Grants 0565399 U, 0635245 N (Cui).
REFERENCES
- 1.Chapleau MW, Hajduczok G, Abboud FM. Paracrine role of prostanoids in activation of arterial baroreceptors: an overview. Clin Exp Hypertens A. 1991;13:817–824. doi: 10.3109/10641969109042085. [DOI] [PubMed] [Google Scholar]
- 2.Chen HI, Chapleau MW, McDowell TS, Abboud FM. Prostaglandins contribute to activation of baroreceptors in rabbits. Possible paracrine influence of endothelium. Circ Res. 1990;67:1394–1404. doi: 10.1161/01.res.67.6.1394. [DOI] [PubMed] [Google Scholar]
- 3.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Zhang R, Wilson TE, Crandall CG. Spectral analysis of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2004;286:H1101–H1106. doi: 10.1152/ajpheart.00790.2003. [DOI] [PubMed] [Google Scholar]
- 5.Davy KP, Herbert WG, Williams JH. Effect of indomethacin on the pressor responses to sustained isometric contraction in humans. J Appl Physiol. 1993;75:273–278. doi: 10.1152/jappl.1993.75.1.273. [DOI] [PubMed] [Google Scholar]
- 6.Doerzbacher KJ, Ray CA. Muscle sympathetic nerve responses to physiological changes in prostaglandin production in humans. J Appl Physiol. 2001;90:624–629. doi: 10.1152/jappl.2001.90.2.624. [DOI] [PubMed] [Google Scholar]
- 7.Dzau VJ, Packer M, Lilly LS, Swartz SL, Hollenberg NK, Williams GH. Prostaglandins in severe congestive heart failure. Relation to activation of the renin-angiotensin system and hyponatremia. N Engl J Med. 1984;310:347–352. doi: 10.1056/NEJM198402093100603. [DOI] [PubMed] [Google Scholar]
- 8.Fagraeus L, Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol. 1976;40:679–682. doi: 10.1152/jappl.1976.40.5.679. [DOI] [PubMed] [Google Scholar]
- 9.Fontana GA, Pantaleo T, Bongianni F, Cresci F, Lavorini F, Guerra CT, Panuccio P. Prostaglandin synthesis blockade by ketoprofen attenuates respiratory and cardiovascular responses to static handgrip. J Appl Physiol. 1995;78:449–457. doi: 10.1152/jappl.1995.78.2.449. [DOI] [PubMed] [Google Scholar]
- 10.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 11.Gordon SM, Brahim JS, Rowan J, Kent A, Dionne RA. Peripheral prostanoid levels and nonsteroidal anti-inflammatory drug analgesia: replicate clinical trials in a tissue injury model. Clin Pharmacol Ther. 2002;72:175–183. doi: 10.1067/mcp.2002.126501. [DOI] [PubMed] [Google Scholar]
- 12.Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol. 2006;290:H2239–H2246. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- 13.Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975;38:272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. Chapter 10. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 381–447. [Google Scholar]
- 15.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of group III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol. 1984;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- 17.Kurita A, Takase B, Hikita H, Uehata A, Nishioka T, Nagayoshi H, Satomura K, Nakao S. Frequency domain heart rate variability and plasma norepinephrine level in the coronary sinus during handgrip exercise. Clin Cardiol. 1999;22:207–212. doi: 10.1002/clc.4960220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee F, Shoemaker JK, McQuillan PM, Kunselman AR, Smith MB, Yang QX, Smith H, Gray K, Sinoway LI. Effects of forearm Bier block with bretylium on the hemodynamic and metabolic responses to handgrip. Am J Physiol Heart Circ Physiol. 2000;279:H586–H593. doi: 10.1152/ajpheart.2000.279.2.H586. [DOI] [PubMed] [Google Scholar]
- 19.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- 20.McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise: Implications for congestive heart failure. J Clin Invest. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol (London) 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol. 2004;287:H1944–H1949. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- 23.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- 24.Monahan KD, Ray CA. Cyclooxygenase inhibition and baroreflex sensitivity in humans. Am J Physiol Heart Circ Physiol. 2005;288:H737–H743. doi: 10.1152/ajpheart.00357.2004. [DOI] [PubMed] [Google Scholar]
- 25.Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol. 1990;259:H745–H750. doi: 10.1152/ajpheart.1990.259.3.H745. [DOI] [PubMed] [Google Scholar]
- 26.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol. 1990;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- 27.Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. Central circulatory adjustments to dynamic exercise. Chapter 5; pp. 162–203. [Google Scholar]
- 28.Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- 29.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- 30.Smith WL. Prostanoid biosynthesis and mechanisms of action. Am J Physiol Renal Physiol. 1992;263:F181–F191. doi: 10.1152/ajprenal.1992.263.2.F181. [DOI] [PubMed] [Google Scholar]
- 31.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 32.Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res. 1986;59:645–654. doi: 10.1161/01.res.59.6.645. [DOI] [PubMed] [Google Scholar]
- 33.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–2039. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 34.Vallbo AB, Hagbarth K–E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 35.Waldrop TG, Rybicki KJ, Kaufman MP, Ordway GA. Activation of visceral thin-fiber afferents increases respiratory output in cats. Respir Physiol. 1984;58:187–196. doi: 10.1016/0034-5687(84)90147-6. [DOI] [PubMed] [Google Scholar]
- 36.Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol Heart Circ Physiol. 1993;265:H171–H175. doi: 10.1152/ajpheart.1993.265.1.H171. [DOI] [PubMed] [Google Scholar]