Abstract
Infections with the filamentous fungus Aspergillus fumigatus are among the most devastating of the systemic mycoses. Unlike most primary pathogens, which possess virulence traits that developed in association with a host organism, evidence suggests that the virulence of A. fumigatus entails a collection of ‘street-smart’ attributes that have evolved to resist the adverse selection pressures encountered in decaying vegetation. These features enhance the overall competitiveness of the organism in its environmental niche, but are also thought to promote growth and survival in a human host. Although many of the genes that are responsible for these characteristics do not fit into the classical definition of a virulence factor, they are nonetheless important to the pathogenesis of aspergillosis and may therefore provide novel opportunities for antifungal development.
Introduction
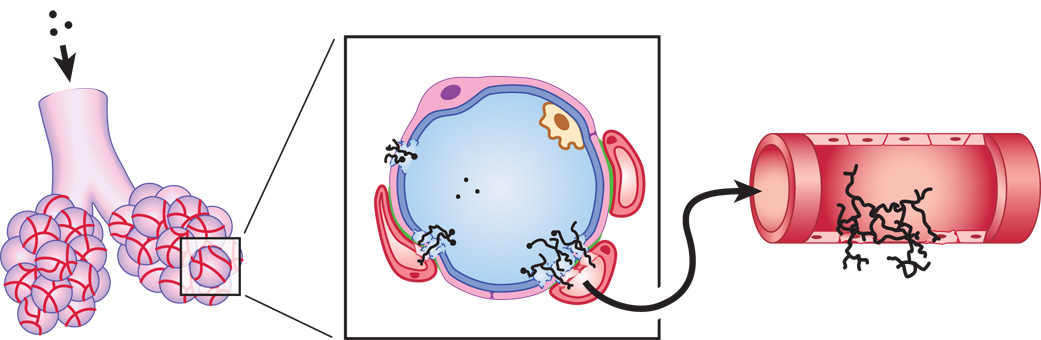
Aspergillus fumigatus is a saprophytic filamentous fungus that is the predominant mold pathogen of the immunosuppressed population [1]. The organism is acquired through the inhalation of asexual spores called conidia, which are widespread in the environment and small enough to reach the distal airways. The conidia are of minimal concern to healthy individuals because they are cleared by pulmonary defenses. However, when the immune system is compromised, the conidia may germinate into hyphae and establish a focus of infection within the lung. Although it is likely that conidial germination begins within the surfactant layer, both conidia and hyphae can be endocytosed by, and grow within, lung epithelial cells [2]. Since the ability of A. fumigatus to assimilate nutrients from a complex substrate requires the secretion of extracellular hydrolases [3], the progressive growth of hyphae within the lung eventually damages the epithelial barrier, providing access to the interalveolar septum where the fungus can enter the vasculature by penetrating endothelial cells (Fig. 1). Hyphal fragments are then free to migrate to distal sites, and the prognosis for disseminated infection is very poor [4,5]. Throughout the infection, A. fumigatus must continually adjust its physiology to survive in the host environment, and the genes that have been implicated in this adaptability are the subject of this review. The emphasis is on fungal gene products since the contribution of the host has been highlighted in several recent articles [6–8].
Fig. 1.
Schematic illustration of the pathogenesis of invasive aspergillosis (not drawn to scale). A. fumigatus conidia are small enough (2-3 µm in diameter) to reach the distal airways when inhaled (left). A cross section of the alveolar space is enlarged in the center panel, showing the close proximity of adjacent blood vessels in the interalveolar septum. In a susceptible host, the conidia are able to germinate and damage the blood-air interface. This barrier is comprised of a surfactant layer (blue), a type I pulmonary epithelial cell (pink), and an underlying microvascular endothelial cell (red). Loose interstitial tissue can sometimes be found between the epithelial and endothelial cells, but when the two cells are closely apposed the basal laminae fuse (green), making the barrier only 0.1 – 1.5 µm in thickness. The growing hyphae eventually penetrate this barrier and hyphal fragments are released into the blood, providing access to other organs by extravascular invasion (right panel).
Sustaining growth at body temperature
A. fumigatus resides in compost, a dynamic environment that undergoes wide fluctuations in temperature as a consequence of intense microbial activity. The ability of A. fumigatus to thrive in this niche requires a substantial level of thermotolerance that has been speculated to contribute to virulence [9–11]. Although A. fumigatus displays a distinct pattern of gene expression at 37°C [12], only three genes have been demonstrated to be necessary for thermotolerant growth: the ribosome biogenesis protein CgrA [13], the O-mannosyltransferase Pmt1 [14] and a protein of unknown function, ThtA [15]. Disruption of any of these genes influences thermotolerance to some degree. However, only the ΔcgrA mutant has impaired growth and virulence at 37°C. The ribosome defect in the ΔcgrA mutant is present at 22°C, even though the mutant grows normally at this temperature [16]. This suggests that the defect in ribosome biogenesis caused by loss of CgrA is compatible with the limited physiological demands at low temperature but not with the heightened metabolic requirements at 37°C. The challenge for the future will be to determine whether the thermotolerance of A. fumigatus can be reduced further by disrupting other genes involved in ribosome biogenesis, with the goal of rendering the organism incapable of growth at 37°C.
Maintaining a rigid yet permeable barrier to the environment
In addition to providing structural integrity, the cell wall represents the major interface between the internal physiology of the fungus and the hostile environment of either compost or human tissue. The wall of A. fumigatus is comprised of branched and linear β-(1,3) and β-(1,4) glucan, α-(1,3) glucan, chitin, chitosan and galactomannan [17]. There are three predicted α-(1,3) glucan synthase genes in the A. fumigatus genome, but only a disruption of ags1 resulted in a decrease in cell wall α-(1,3) glucan [18]. The Δags1 mutant retained virulence, despite suffering a 50% reduction in α-(1,3) glucan content, indicating that the fungus can tolerate a considerable loss of this polysaccharide and still maintain cell wall homeostasis. Surprisingly, deletion of ags3 increased virulence without affecting α-(1,3) glucan content [18]. The normal level of α-(1,3) glucan in this mutant is likely to be a consequence of redundancy between ags1 and ags3 since ags1 levels were dramatically upregulated in the Δags3 strain. The hypervirulence of Δags3 was speculated to involve the observed increase in melanin, resistance to oxidative stress and more rapid germination of the mutant conidia, although the mechanism by which loss of Ags3 induces this phenotype remains to be elucidated.
Chitin is a polymer of N-acetyl-glucosamine that confers high tensile strength upon the wall [19]. Of the three chitin synthase genes that have been examined in A. fumigatus, only the double ΔchsC/ΔchsG mutant showed a reduction in virulence, a phenotype that was attributed to chsG because the ΔchsG mutant had the same morphological abnormalities as the ΔchsC/ΔchsG mutant [20]. At least seven chitin synthase genes can be found in the A. fumigatus, suggesting that considerable redundancy among these proteins has evolved to ensure that cell wall homeostasis is maintained when the organism is confronted with adverse conditions that interfere with wall integrity.
Glycophosphatidyl-inositol (GPI)-linked proteins anchored to the plasma membrane also play important roles in fungal cell wall organization. The family of GPI-anchored β-1,3-glucanosyltransferases are thought to participate in the elongation of β(1-3) side chains in the A. fumigatus cell wall, and at least one of these genes, gel2, is required to support the virulence of A. fumigatus [21]. By contrast, deletion of the GPI-linked Ecm33 protein increased virulence, possibly resulting from the increased germination rate of this mutant [22]. A more global block of GPI-anchored protein function was accomplished by disrupting Afpig-a, encoding the catalytic subunit of an enzyme involved in GPI anchor biosynthesis [23]. The absence of this protein was associated with reduced cell wall integrity and attenuated virulence, demonstrating the importance of this general protein class in both cell wall function and pathogenesis.
The wall of A. fumigatus conidia is distinguished by the presence of melanin, a pigment that is thought to defend the genome from the adverse effects of ionizing radiation in the environment. Pigment biosynthesis is catalyzed by a polyketide synthase, and mutants lacking this enzyme have white conidia and attenuated virulence when inoculated intravenously into mice [24,25]. Pigmentless conidia are more susceptible to phagocytic killing than wild type conidia, which may explain their lack of virulence in this model.
Despite the considerable redundancy among cell wall synthesis genes in A. fumigatus, the wall remains an attractive target for therapy because of its fungal specificity and the fact that an intact cell wall is essential to the organism. The challenge will be to identify new strategies that can disrupt cell wall synthesis in a more global fashion so that overlapping pathways are unable to protect the organism.
Secretion of damaging products
A. fumigatus secretes numerous secondary metabolites into its environment [26], which is thought to provide a chemical shield against competing or predatory species [27,28]. The secondary metabolite gliotoxin has attracted the most interest in A. fumigatus because of its potent immunosuppressive and cytocidal properties and the fact that it can be readily detected during experimental infection and in sera from patients with aspergillosis [29–32]. Two studies have shown that blocking gliotoxin production by disrupting the gliP gene had no effect on virulence in neutropenic mice, arguing against a major role for this toxin in the pathogenesis of aspergillosis [33,34]. However, recent studies have shown that the contribution of gliotoxin to virulence is host strain-dependent and requires an immunosuppression protocol that does not cause neutropenia [35,36]. These results imply that gliotoxin augments virulence only when some neutrophil function is present, raising the possibility that neutrophils are the major target of this toxin. A more global repression of secondary metabolite production was accomplished by disrupting laeA, encoding a predicted protein methyltransferase that regulates the expression of secondary metabolite clusters [37]. Although the ΔlaeA mutant lacked gliotoxin, and was hypovirulent in mice, the mechanism for the attenuated virulence can not be identified with certainty because LaeA influences the expression of ~10% of the A. fumigatus genome [38].
Signaling and responding to stress
The ability of A. fumigatus to reprogram its physiology in response to the environment requires an effective communication strategy that is mediated by signal transduction pathways [39]. The cAMP-dependent protein kinase (PKA) is key to this signaling, particularly with respect to the sensing of carbon source and environmental stress. The central messenger of the pathway is cAMP, produced by the action of adenylate cyclase, an enzyme that is under the regulation of the G protein α-subunit GpaB. Accumulating levels of cAMP bind to the regulatory subunit of PKA, PkaR, thereby liberating the catalytic subunits PkaC1 and PkaC2, which then phosphorylate downstream targets and trigger the appropriate adaptive responses. Dysregulation of the PKA pathway, either by disrupting its activity (ΔgpaB, or ΔpkaC1) [40], or allowing unrestrained PKA activity (ΔpkaR)[41], attenuates virulence in mice. This suggests that an imbalance in A. fumigatus PKA signaling, in either direction, is deleterious to the pathogenesis of aspergillosis, presumably by disrupting the ability of the fungus to sense and adequately respond to host-specific stressors.
Calcineurin is a Ca2+-calmodulin-activated protein phosphatase that is an important mediator of calcium signaling and stress responses in eukaryotic organisms [42]. Deletion of cnaA, encoding the catalytic A subunit of calcineurin, profoundly impaired the growth of A. fumigatus hyphae and rendered the organism almost avirulent in multiple infection models [43,44]. By contrast, a mutant lacking the calcineurin-dependent transcription factor CrzA grew normally in vitro, but was still attenuated for virulence [45]. These findings argue that the impaired virulence of the ΔcnaA mutant is not simply due to reduced hyphal growth. Since calcineurin inhibitors are aready in use for the treatment of other diseases, it may be possible to manipulate this pathway to improve outcome in patients with invasive aspergillosis.
Virulence studies have been reported on one of the four mitogen-activated (MAP) kinases in the A. fumigatus genome, mpkA [46]. Despite a heightened sensitivity to cell wall stress, and a considerable growth defect on standard laboratory medium, the ΔmpkA mutant was as virulent as wild type A. fumigatus. Interestingly, deletion of an upstream regulator of the high osmolarity glycerol (HOG)-MAPK pathway, sho1, also impaired in vitro grow rate without affecting virulence [47]. The lack of correlation between in vitro growth rate and virulence in the ΔmpkA and Δsho1 mutants suggest that using growth rate to predict virulence potential is perhaps an oversimplification of a complex phenotype.
Oxidative damage generated by host immune responses is one of the major sources of stress encountered by pathogens. Although infections with A. fumigatus occur primarily in immunocompromised patients, the severity of the immunosuppression varies widely, so it is likely that residual host defenses can influence the progression of the disease. Several A. fumigatus genes have been reported to modify the sensitivity of the organism to oxidative stress, including catalases (catA, cat1, cat2)[48,49], the PKA regulatory subunit PkaR [41], the MAPK pathway (mpkA, sho1)[46,47], and two transcription factors that mediate oxidative stress responses (yap1 and skn7)[50]. However, only the ΔpkaR and Δcat1/Δcat2 mutants showed a reduction in virulence. These results argue against a major role for these anti-oxidant responses in an immunocompromised host, although it is conceivable that they could influence virulence under conditions of less severe host immunosuppression, analogous to the findings reported for the gliotoxin mutant [35,36].
Meeting nutritional requirements
In order to compete effectively in the environment, A. fumigatus must be able to adjust its metabolism to ensure that its needs are met during periods of fluctuating nutrient availability. Accumulating evidence indicates that this metabolic versatility is of particular importance in the host environment. One of the most striking examples of this is illustrated by iron. A. fumigatus uses siderophores for both iron acquisition and intracellular iron storage [51], and disrupting the siderophore biosynthetic pathway at multiple steps has revealed that A. fumigatus relies heavily on these low-molecular weight chelators for growth in the iron-limited environment of the host [51–53]. Zinc also has limited bioavailability in vivo, and the zinc-responsive transcriptional activator ZafA is required to support virulence, presumably by enhancing zinc uptake mechanisms [54].
Several studies have shown that mutants of A. fumigatus that are auxotrophic for p-aminobenzoic acid [55–57], uridine/uracil [58] or lysine [59] are avirulent, suggesting that these nutrients can not be acquired in sufficient quantities from host tissues, at least during the initial part of the infection. A nitrogen source is also required for fungal growth, and the transcription factor AreA and the Ras-related protein RhbA have been implicated as signaling molecules that respond to nitrogen availability [60–62]. The ΔareA and ΔrhbA mutants are both hypovirulent in mice, suggesting that the nitrogen that is available in the host environment may require one or both of these proteins for optimal utilization by the fungus. The CpcA transcriptional activator of the cross-pathway control (CPC) system of amino acid biosynthesis is also required for the virulence of A. fumigatus [63]. This suggests that available amino acid pools in the host may be imbalanced or, alternatively, that CpcA influences the expression of one or more virulence factors. Surprisingly, deleting the upstream signaling sensor kinase of the CPC system, CpcC, was dispensable for pathogenicity [64]. Since CpcC is responsible for upregulating the CPC pathway in response to stress, it appears that basal expression of the CPC system, rather than induced expression, supports virulence. It will be of interest to identify the target genes that are downstream of both CpcA and CpcC signaling.
A. fumigatus must continually extract nutrients from host tissues throughout the infection, which requires the secretion of degradative enzymes such as proteases. Deletion of several genes encoding secreted proteases has yet to demonstrate a role for such enzymes in virulence [65]. However, it is premature to discount the importance of protease secretion since only a limited number of them have been explored to date, and at least 99 secreted proteases are predicted in the A. fumigatus genome [12,66]. Nevertheless, the progressive destruction of host tissues by A. fumigatus is likely to release amino acids that can be used by the fungus to support growth. An adverse consequence of amino acid metabolism is the toxic accumulation of propionyl-CoA, a problem that is countered by methylcitrate synthase, McsA, involved in the methylcitrate cycle [67,68]. The striking reduction in virulence of a ΔmcsA mutant suggests that A. fumigatus relies upon protein degradation as a food source in vivo, making the fungus vulnerable to propionyl-coA accumulation [67,68]. Since fungi and mammals handle propionyl-coA metabolism differently, an important implication of this finding is that it may be possible to design strategies to selectively interrupt the fungal pathway. Taken together, each of these metabolic studies has revealed that, although the nutritional environment of the host is not ideal for A. fumigatus, the fungus is well equipped to optimize its metabolism for the utilization of host tissues as a food supply. Further understanding of metabolic traits that are required for virulence may offer exciting new prospects for antifungal development.
Conclusions
It is becoming increasingly clear that the virulence of A. fumigatus is multifactorial, involving networks of genes that have likely evolved to support the organism in its primary ecological niche. The functions of these genes are diverse, influencing the integrity of the cell wall, the signaling pathways that detect and respond to environmental changes, and the adaptive responses that enhance overall fitness, most notably in the area of nutritional versatility. Although we have gained important insights into aspects of fungal physiology that support the growth of A. fumigatus in the host, the virulence determinants identified so far are not unique to this species and we have yet to determine what makes this fungus a more potent opportunistic pathogen than other commonly encountered environmental molds. The challenge for future research will be to obtain a comprehensive understanding of the requirements of this fungus in the host environment so that more effective strategies can be developed to interrupt these pathways.
Acknowledgments
The author is supported by funds from the NIH and the Cystic Fibrosis Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67:1567–1601. doi: 10.2165/00003495-200767110-00004. [DOI] [PubMed] [Google Scholar]
- 2.Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006;2:e129. doi: 10.1371/journal.ppat.0020129.• An excellent review of the mechanisms by which A. fumigatus and other fungal pathogens invade host cells that are not professional phagocytes.
- 3.Tekaia F, Latge JP. Aspergillus fumigatus: saprophyte or pathogen? Curr Opin Microbiol. 2005;8:385–392. doi: 10.1016/j.mib.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 5.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 6.Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell. 2007;6:1953–1963. doi: 10.1128/EC.00274-07.• An excellent review of A. fumigatus pathogenesis, integrating data from the perspective of both the fungus and the host response.
- 7.Rivera A, Hohl T, Pamer EG. Immune responses to Aspergillus fumigatus infections. Biol Blood Marrow Transplant. 2006;12:47–49. doi: 10.1016/j.bbmt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Montagnoli C, Bozza S, Gaziano R, Zelante T, Bonifazi P, Moretti S, Bellocchio S, Pitzurra L, Romani L. Immunity and tolerance to Aspergillus fumigatus. Novartis Found Symp. 2006;279:66–77. discussion 77-69, 216-219. [PubMed] [Google Scholar]
- 9.Araujo R, Rodrigues AG. Variability of germinative potential among pathogenic species of Aspergillus. J Clin Microbiol. 2004;42:4335–4337. doi: 10.1128/JCM.42.9.4335-4337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhabhra R, Askew DS. Thermotolerance and virulence of Aspergillus fumigatus: role of the fungal nucleolus. Med Mycol. 2005;43 Suppl 1:S87–S93. doi: 10.1080/13693780400029486. [DOI] [PubMed] [Google Scholar]
- 11.Beffa T, Staib F, Lott Fischer J, Lyon PF, Gumowski P, Marfenina OE, Dunoyer-Geindre S, Georgen F, Roch-Susuki R, Gallaz L, et al. Mycological control and surveillance of biological waste and compost. Med Mycol. 1998;36 Suppl 1:137–145. [PubMed] [Google Scholar]
- 12.Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332.•• This landmark paper reports the genomic sequence of A. fumigatus, and provides the first microarray analysis of temperature-regulated gene expression in this mold.
- 13.Bhabhra R, Miley MD, Mylonakis E, Boettner D, Fortwendel J, Panepinto JC, Postow M, Rhodes JC, Askew DS. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect Immun. 2004;72:4731–4740. doi: 10.1128/IAI.72.8.4731-4740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Hu H, Zhang L, Li R, Ouyang H, Ming J, Jin C. O-Mannosyltransferase 1 in Aspergillus fumigatus (AfPmt1p) is crucial for cell wall integrity and conidium morphology, especially at an elevated temperature. Eukaryot Cell. 2007;6:2260–2268. doi: 10.1128/EC.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YC, Tsai HF, Karos M, Kwon-Chung KJ. THTA, a thermotolerance gene of Aspergillus fumigatus. Fungal Genet Biol. 2004;41:888–896. doi: 10.1016/j.fgb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Bhabhra R, Richie DL, Kim HS, Nierman WC, Fortwendel J, Aris JP, Rhodes JC, Askew DS. Impaired ribosome biogenesis disrupts the integration between morphogenesis and nuclear duplication during the germination of Aspergillus fumigatus. Eukaryot Cell. 2008 doi: 10.1128/EC.00412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latge JP, Mouyna I, Tekaia F, Beauvais A, Debeaupuis JP, Nierman W. Specific molecular features in the organization and biosynthesis of the cell wall of Aspergillus fumigatus. Med Mycol. 2005;43 Suppl 1:S15–S22. doi: 10.1080/13693780400029155. [DOI] [PubMed] [Google Scholar]
- 18.Maubon D, Park S, Tanguy M, Huerre M, Schmitt C, Prevost MC, Perlin DS, Latge JP, Beauvais A. AGS3, an alpha(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet Biol. 2006;43:366–375. doi: 10.1016/j.fgb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Roncero C. The genetic complexity of chitin synthesis in fungi. Curr Genet. 2002;41:367–378. doi: 10.1007/s00294-002-0318-7. [DOI] [PubMed] [Google Scholar]
- 20.Mellado E, Aufauvre-Brown A, Gow NA, Holden DW. The Aspergillus fumigatus chsC and chsG genes encode class III chitin synthases with different functions. Mol Microbiol. 1996;20:667–679. doi: 10.1046/j.1365-2958.1996.5571084.x. [DOI] [PubMed] [Google Scholar]
- 21.Mouyna I, Morelle W, Vai M, Monod M, Lechenne B, Fontaine T, Beauvais A, Sarfati J, Prevost MC, Henry C, et al. Deletion of GEL2 encoding for a beta(1-3)glucanosyltransferase affects morphogenesis and virulence in Aspergillus fumigatus. Mol Microbiol. 2005;56:1675–1688. doi: 10.1111/j.1365-2958.2005.04654.x. [DOI] [PubMed] [Google Scholar]
- 22.Romano J, Nimrod G, Ben-Tal N, Shadkchan Y, Baruch K, Sharon H, Osherov N. Disruption of the Aspergillus fumigatus ECM33 homologue results in rapid conidial germination, antifungal resistance and hypervirulence. Microbiology. 2006;152:1919–1928. doi: 10.1099/mic.0.28936-0. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Zhou H, Luo Y, Ouyang H, Hu H, Jin C. Glycosylphosphatidylinositol (GPI) anchor is required in Aspergillus fumigatus for morphogenesis and virulence. Mol Microbiol. 2007;64:1014–1027. doi: 10.1111/j.1365-2958.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- 24.Langfelder K, Jahn B, Gehringer H, Schmidt A, Wanner G, Brakhage AA. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med Microbiol Immunol. 1998;187:79–89. doi: 10.1007/s004300050077. [DOI] [PubMed] [Google Scholar]
- 25.Tsai HF, Chang YC, Washburn RG, Wheeler MH, Kwon-Chung KJ. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol. 1998;180:3031–3038. doi: 10.1128/jb.180.12.3031-3038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keller NP, Turner G, Bennett JW. Fungal secondary metabolism - from biochemistry to genomics. Nat Rev Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286.• An excellent review of fungal secondary metabolism, emphasizing the different classes of metabolites and their regulation by genetic and epigenetic mechanisms.
- 27.Rohlfs M, Albert M, Keller NP, Kempken F. Secondary chemicals protect mould from fungivory. Biol Lett. 2007;3:523–525. doi: 10.1098/rsbl.2007.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardiner DM, Waring P, Howlett BJ. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology. 2005;151:1021–1032. doi: 10.1099/mic.0.27847-0. [DOI] [PubMed] [Google Scholar]
- 29.Lewis RE, Wiederhold NP, Chi J, Han XY, Komanduri KV, Kontoyiannis DP, Prince RA. Detection of gliotoxin in experimental and human aspergillosis. Infect Immun. 2005;73:635–637. doi: 10.1128/IAI.73.1.635-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullbacher A, Eichner RD. Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proc Natl Acad Sci U S A. 1984;81:3835–3837. doi: 10.1073/pnas.81.12.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullbacher A, Waring P, Eichner RD. Identification of an agent in cultures of Aspergillus fumigatus displaying anti-phagocytic and immunomodulating activity in vitro. J Gen Microbiol. 1985;131:1251–1258. doi: 10.1099/00221287-131-5-1251. [DOI] [PubMed] [Google Scholar]
- 32.Pardo J, Urban C, Galvez EM, Ekert PG, Muller U, Kwon-Chung J, Lobigs M, Mullbacher A, Wallich R, Borner C, et al. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J Cell Biol. 2006;174:509–519. doi: 10.1083/jcb.200604044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cramer RA, Jr, Gamcsik MP, Brooking RM, Najvar LK, Kirkpatrick WR, Patterson TF, Balibar CJ, Graybill JR, Perfect JR, Abraham SN, et al. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell. 2006;5:972–980. doi: 10.1128/EC.00049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kupfahl C, Heinekamp T, Geginat G, Ruppert T, Hartl A, Hof H, Brakhage AA. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol. 2006;62:292–302. doi: 10.1111/j.1365-2958.2006.05373.x. [DOI] [PubMed] [Google Scholar]
- 35.Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, Mullbacher A, Gallin JI, Simon MM, Kwon-Chung KJ. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell. 2007;6:1562–1569. doi: 10.1128/EC.00141-07.• This is the first paper to resolve the conflicting data in the literature regarding the role of gliotoxin in the virulence of A. fumigatus. The data reveal that the contribution of gliotoxin to virulence is host strain-dependent and requires an immunosuppression protocol that does not cause neutropenia.
- 36.Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, Ejzykowicz DE, Chiang LY, Filler SG, May GS. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis. 2008;197:479–486. doi: 10.1086/525044. [DOI] [PubMed] [Google Scholar]
- 37.Bok JW, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Keller NP. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell. 2005;4:1574–1582. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrin RM, Fedorova ND, Bok JW, Cramer RA, Wortman JR, Kim HS, Nierman WC, Keller NP. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007;3:e50. doi: 10.1371/journal.ppat.0030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahn YS, Xue C, Idnurm A, Rutherford JC, Heitman J, Cardenas ME. Sensing the environment: lessons from fungi. Nat Rev Microbiol. 2007;5:57–69. doi: 10.1038/nrmicro1578. [DOI] [PubMed] [Google Scholar]
- 40.Liebmann B, Muller M, Braun A, Brakhage AA. The cyclic AMP-dependent protein kinase a network regulates development and virulence in Aspergillus fumigatus. Infect Immun. 2004;72:5193–5203. doi: 10.1128/IAI.72.9.5193-5203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao W, Panepinto JC, Fortwendel JR, Fox L, Oliver BG, Askew DS, Rhodes JC. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect Immun. 2006;74:4865–4874. doi: 10.1128/IAI.00565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox DS, Heitman J. Good fungi gone bad: the corruption of calcineurin. Bioessays. 2002;24:894–903. doi: 10.1002/bies.10157. [DOI] [PubMed] [Google Scholar]
- 43.Steinbach WJ, Cramer RA, Jr, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK, Jr, Heitman J, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:1091–1103. doi: 10.1128/EC.00139-06.• This study provides the first report of the phenotype of a calcineurin-deficient mutant. The data provide strong evidence that this pathway would have considerable merit as an antifungal target in A. fumigatus
- 44.da Silva Ferreira ME, Heinekamp T, Hartl A, Brakhage AA, Semighini CP, Harris SD, Savoldi M, de Gouvea PF, de Souza Goldman MH, Goldman GH. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet Biol. 2007;44:219–230. doi: 10.1016/j.fgb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Soriani FM, Malavazi I, da Silva Ferreira ME, Savoldi M, Von Zeska Kress MR, de Souza Goldman MH, Loss O, Bignell E, Goldman GH. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol. 2008;67:1274–1291. doi: 10.1111/j.1365-2958.2008.06122.x.•• This paper demonstrates a role for the calcineurin-dependent transcription factor CrzA in the stress response and virulence of A. fumigatus. In contrast to the calcineurin mutant ΔcnaA, which showed a severe growth defect in association with attenuated virulence, the ΔcrzA mutant had attenuated virulence despite a normal growth rate. These findings argue that impaired growth may not be the sole effector of reduced virulence of mutants in this pathway.
- 46.Valiante V, Heinekamp T, Jain R, Hartl A, Brakhage AA. The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet Biol. 2007 doi: 10.1016/j.fgb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Qiao J, Liu W, Wan Z, Wang X, Calderone R, Li R. The Sho1 sensor regulates growth, morphology, and oxidant adaptation in Aspergillus fumigatus but is not essential for development of invasive pulmonary aspergillosis. Infect Immun. 2008 doi: 10.1128/IAI.01507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paris S, Wysong D, Debeaupuis JP, Shibuya K, Philippe B, Diamond RD, Latge JP. Catalases of Aspergillus fumigatus. Infect Immun. 2003;71:3551–3562. doi: 10.1128/IAI.71.6.3551-3562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calera JA, Paris S, Monod M, Hamilton AJ, Debeaupuis JP, Diaquin M, Lopez-Medrano R, Leal F, Latge JP. Cloning and disruption of the antigenic catalase gene of Aspergillus fumigatus. Infect Immun. 1997;65:4718–4724. doi: 10.1128/iai.65.11.4718-4724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lessing F, Kniemeyer O, Wozniok I, Loeffler J, Kurzai O, Haertl A, Brakhage AA. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the Current major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot Cell. 2007;6:2290–2302. doi: 10.1128/EC.00267-07.• This paper is the first to use a proteomic approach for target gene identification, providing a rational basis subsequent gene deletion. The results demonstrated that although the A. fumigatus Yap1 transcriptional regulator is a major defense against oxidative stress, it is dispensable for virulence. These data challenge the notion that oxidative stress influences the virulence of A. fumigatus in an immunosuppressed mouse model.
- 51.Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, Wallner A, Arst HN, Jr, Haynes K, Haas H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007;3:1195–1207. doi: 10.1371/journal.ppat.0030128.•• This paper provides a comprehensive analysis of the siderophore biosynthetic pathway in A. fumigatus. The data reveal distinct roles for intracellular and extracellular siderophores in germination, sporulation, oxidative stress response and virulence.
- 52.Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN, Jr, Haynes K, Haas H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med. 2004;200:1213–1219. doi: 10.1084/jem.20041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect Immun. 2005;73:5493–5503. doi: 10.1128/IAI.73.9.5493-5503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno MA, Ibrahim-Granet O, Vicentefranqueira R, Amich J, Ave P, Leal F, Latge JP, Calera JA. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol Microbiol. 2007;64:1182–1197. doi: 10.1111/j.1365-2958.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- 55.Tang CM, Smith JM, Arst HN, Jr, Holden DW. Virulence studies of Aspergillus nidulans mutants requiring lysine or p-aminobenzoic acid in invasive pulmonary aspergillosis. Infect Immun. 1994;62:5255–5260. doi: 10.1128/iai.62.12.5255-5260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandhu DK, Sandhu RS, Khan ZU, Damodaran VN. Conditional virulence of a p-aminobenzoic acid-requiring mutant of Aspergillus fumigatus. Infect Immun. 1976;13:527–532. doi: 10.1128/iai.13.2.527-532.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown JS, Aufauvre-Brown A, Brown J, Jennings JM, Arst H, Jr, Holden DW. Signature-tagged and directed mutagenesis identify PABA synthetase as essential for Aspergillus fumigatus pathogenicity. Mol Microbiol. 2000;36:1371–1380. doi: 10.1046/j.1365-2958.2000.01953.x. [DOI] [PubMed] [Google Scholar]
- 58.D'Enfert C, Diaquin M, Delit A, Wuscher N, Debeaupuis JP, Huerre M, Latge JP. Attenuated virulence of uridine-uracil auxotrophs of Aspergillus fumigatus. Infect Immun. 1996;64:4401–4405. doi: 10.1128/iai.64.10.4401-4405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebmann B, Muhleisen TW, Muller M, Hecht M, Weidner G, Braun A, Brock M, Brakhage AA. Deletion of the Aspergillus fumigatus lysine biosynthesis gene lysF encoding homoaconitase leads to attenuated virulence in a low-dose mouse infection model of invasive aspergillosis. Arch Microbiol. 2004;181:378–383. doi: 10.1007/s00203-004-0667-3. [DOI] [PubMed] [Google Scholar]
- 60.Hensel M, Arst HN, Jr, Aufauvre-Brown A, Holden DW. The role of the Aspergillus fumigatus areA gene in invasive pulmonary aspergillosis. Mol Gen Genet. 1998;258:553–557. doi: 10.1007/s004380050767. [DOI] [PubMed] [Google Scholar]
- 61.Panepinto JC, Oliver BG, Fortwendel JR, Smith DL, Askew DS, Rhodes JC. Deletion of the Aspergillus fumigatus gene encoding the Ras-related protein RhbA reduces virulence in a model of Invasive pulmonary aspergillosis. Infect Immun. 2003;71:2819–2826. doi: 10.1128/IAI.71.5.2819-2826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panepinto JC, Oliver BG, Amlung TW, Askew DS, Rhodes JC. Expression of the Aspergillus fumigatus rheb homologue, rhbA, is induced by nitrogen starvation. Fungal Genet Biol. 2002;36:207–214. doi: 10.1016/s1087-1845(02)00022-1. [DOI] [PubMed] [Google Scholar]
- 63.Krappmann S, Bignell EM, Reichard U, Rogers T, Haynes K, Braus GH. The Aspergillus fumigatus transcriptional activator CpcA contributes significantly to the virulence of this fungal pathogen. Mol Microbiol. 2004;52:785–799. doi: 10.1111/j.1365-2958.2004.04015.x. [DOI] [PubMed] [Google Scholar]
- 64.Sasse C, Bignell EM, Hasenberg M, Haynes K, Gunzer M, Braus GH, Krappmann S. Basal expression of the Aspergillus fumigatus transcriptional activator CpcA is sufficient to support pulmonary aspergillosis. Fungal Genet Biol. 2008 doi: 10.1016/j.fgb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Rementeria A, Lopez-Molina N, Ludwig A, Vivanco AB, Bikandi J, Ponton J, Garaizar J. Genes and molecules involved in Aspergillus fumigatus virulence. Rev Iberoam Micol. 2005;22:1–23. doi: 10.1016/s1130-1406(05)70001-2. [DOI] [PubMed] [Google Scholar]
- 66.Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto K, Arima T, Akita O, Kashiwagi Y, et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 67.Ibrahim-Granet O, Dubourdeau M, Latge JP, Ave P, Huerre M, Brakhage AA, Brock M. Methylcitrate synthase from Aspergillus fumigatus is essential for manifestation of invasive aspergillosis. Cell Microbiol. 2008;10:134–148. doi: 10.1111/j.1462-5822.2007.01025.x.•• This paper demonstrates that the methylcitrate synthase cycle is required for virulence of A. fumigatus, suggesting that host proteins are a major source of nutrients for the fungus during infection.
- 68.Maerker C, Rohde M, Brakhage AA, Brock M. Methylcitrate synthase from Aspergillus fumigatus. Propionyl-CoA affects polyketide synthesis, growth and morphology of conidia. Febs J. 2005;272:3615–3630. doi: 10.1111/j.1742-4658.2005.04784.x. [DOI] [PubMed] [Google Scholar]