Abstract
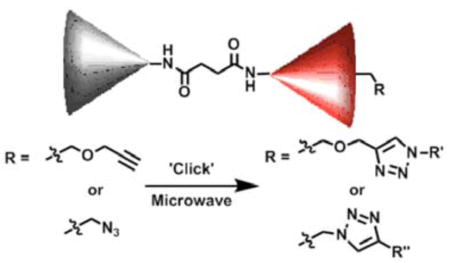
Monofunctionalized polyamide-based dendrimers containing either a terminal azide or alkyne moiety have been designed and synthesized via a convergent synthetic approach. The monofunctionalization allows for the single attachment of a functional moiety in quantitative yields using 1,3 dipolar cycloadditions thereby opening the possibility for targeted dendrimer functionalization.
Dendrimers are important materials for biological applications as a result of their unique features such as the precise control of size and shape, uncommon physical properties, and the placement of numerous functional groups on the periphery and/or core.1 Polyamide-based dendrimers are of particular interest because they are based solely on a peptide like amide backbone and have demonstrated low toxicities and non-immunogenicities.2 Accordingly, polyamide- and polyamidoamine-based dendrimers have been used extensively as biomaterials in gene and drug delivery3 and for nanoparticle encapsulation4 in imaging.5 Although dendrimers containing a single functionality have been reported,6 few of these examples are using the important polyamide backbone. Furthermore, one desired property that has not been realized to date for dendrimers is the possibility to monofunctionalize them selectively in quantitative yields thereby allowing for the single attachment of biological specific targeting and recognition moieties onto the dendrimer surface. Such a strategy would allow for the transportation of the dendrimer to the biological target of interest via a specific binding process and the targeted delivery of drugs or imaging moieties. The main prerequisite for such a functionalization is that the chemical handle for the attachment has to be high yielding and chemoselective. Furthermore, the transformation of choice must allow for the reaction to take place under physiological conditions. One chemical transformation that fulfills these requirements is the 1,3-dipolar cycloaddition between an azide and an alkyne.7,8 Herein, we present the first synthesis of polyamide-based dendrimers that contain a single alkyne or azide group on the dendrimer surface and can be monofunctionalized via 1,3 dipolar cycloaddition chemistry in quantitative yields.9
Our research design is based on the convergent synthetic approach and some recent building block developments by the Newkome group.10 Newkome and coworkers reported the syntheses of the nonfunctionalized dendrons C and D shown in Figure 1 (building block C is also known as Behera’s amine).11 Furthermore, they introduced 1→ (2+1) C-branching monomers and monofunctionalized dendron monomers that are ideal for our purpose to prepare monofunctionalized dendrimers with peptide like amide backbones and a single chemoselective handle (Figure 1).
Figure 1.
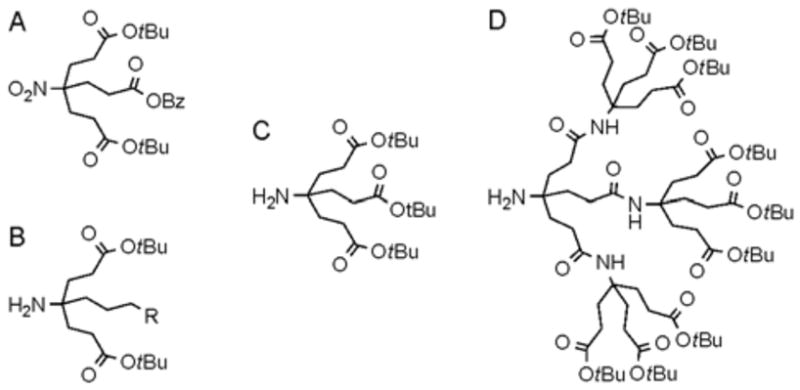
Newkome type monomers; 1→ (2+1) C-branching monomer (A), monofunctionalized monomer (B), and the first (C) and the second (D) generation of nonfunctionalized monomers.
The key building block in our design that will introduce the single functionality onto the dendrimer surface, monomer 3, was prepared by a Michael type addition using 6-nitrohexanol12 and an excess of t-butyl acrylate in the presence of Triton-B to provide monomer 1 in 55% yield (Scheme 1) followed by the protection of the hydroxyl group with Ac2O in pyridine and the catalytic reduction of the nitro group under 60 psi of hydrogen in EtOH. The quantitative reduction of the nitro group to the corresponding amine was supported by the chemical shift (13C NMR) for the nitro-carbon from 92.8 ppm to 52.5 ppm, respectively. Other building blocks (A, C, and D) were prepared as reported by Newkome and coworkers.10, 11
Scheme 1.
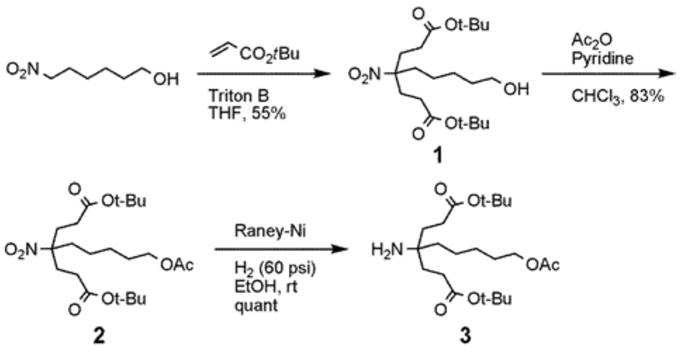
Synthesis of monofunctionalized G1 dendron.
With the basic building blocks in hand, we prepared the second generation of the monofunctionalized dendrons via the selective deprotection of the tert-butyl groups of A followed by the amidation coupling with two equivalents of C (Scheme 2) to afford compound 4 in 87% isolated yield. After the deprotection of the benzyl ester group using Pd/C under 60 psi of hydrogen at room temperature for 12 h, subsequent amidation reaction of the monoacid species with 3 yielded 5 in 93% yield. The nitro group reduction of 5 with Raney-Ni at 55 °C for 24 h under 60 psi of hydrogen provided the desired second generation dendron 6 in 98% yield which was identified by the chemical shift change for C4° moiety from 92.5 ppm to 52.6 ppm as well as the molecular ion peak (HRMS MALDI-TOF) at m/z 1439.9455 [M + H]+. The overall yield of dendron 6 from its building blocks was 72%.
Scheme 2.
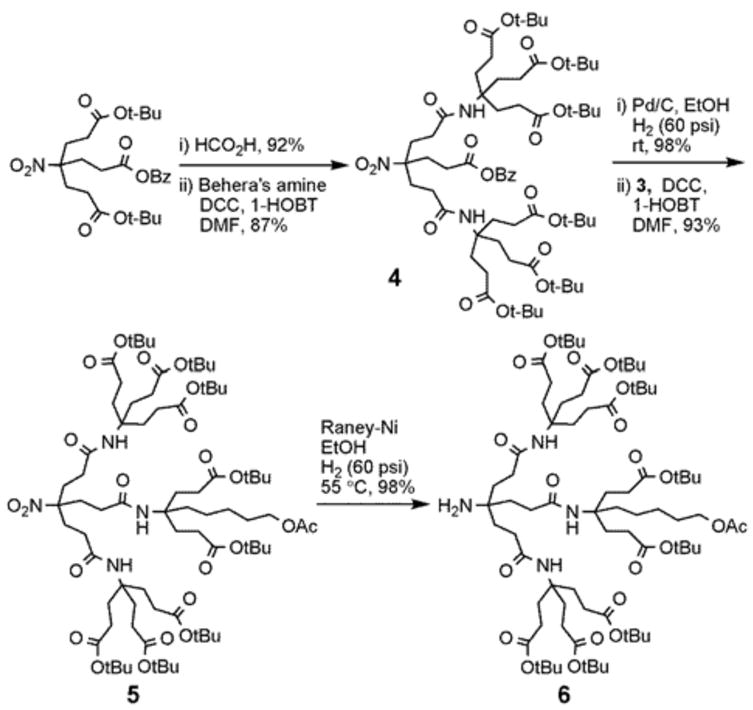
Synthesis of monofunctionalized G2 dendron.
Scheme 3 outlines the synthesis of the monofunctionalized dendrimers 10 and 11 containing the single chemical handle. The synthesis commences with the stepwise incorporation of D and 6 onto the core molecule. The treatment of D with succinic anhydride in pyridine afforded the half shell of dendrimer 7 with an acid moiety at the core. Dendron 6 was then coupled onto the core using DCC and 1-HOBT to provide the protected monofunctionalized dendrimer 8 in 84% yield. Deprotection using K2CO3 in a mixture of MeOH and H2O (10:1) at room temperature for 2 h provided the hydroxyl dendrimer 9 in 92% yield.
Scheme 3.
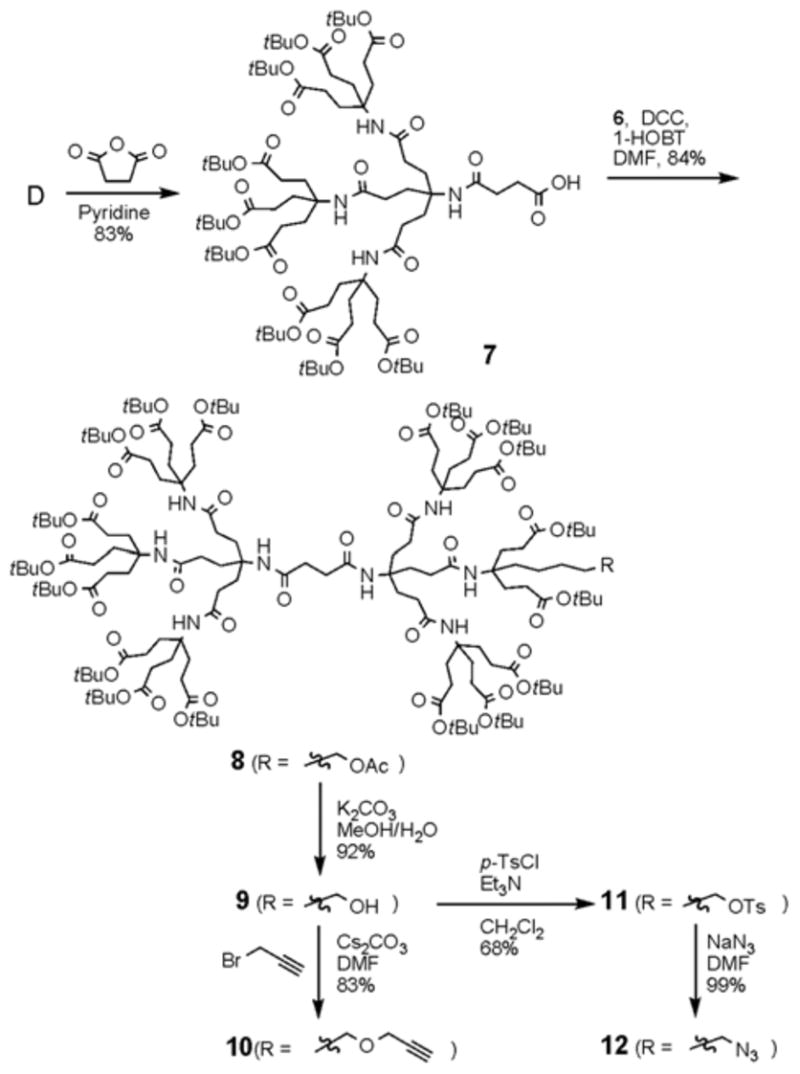
Synthesis of monofunctionalized dendrimers (G2).
For our purposes, both acetylene and azide were introduced onto the dendrimer at the hydroxyl moiety. Dendrimer 9 was treated with propargyl bromide in the presence of Cs2CO3 in DMF at 80 °C for three days to afford the desired acetylenic dendrimer 10 in 83% isolated yield. The transformation was demonstrated by the observation of a new 1H NMR signal at 2.54 ppm for the terminal acetylene proton and two new 13C NMR signals at 75.7 and 77.8 ppm for acetylene carbons.
To obtain the target azide dendrimer 12, 9 was tosylated and subsequently treated with sodium azide in DMF to give 12 in quantitative yields. The conversions were confirmed by the observation of the chemical shift change (1H NMR) for the methylene protons (CH2R) from 3.60 ppm (R = OH) to 3.97 ppm (R = OTs) and 3.26 ppm (R = N3).
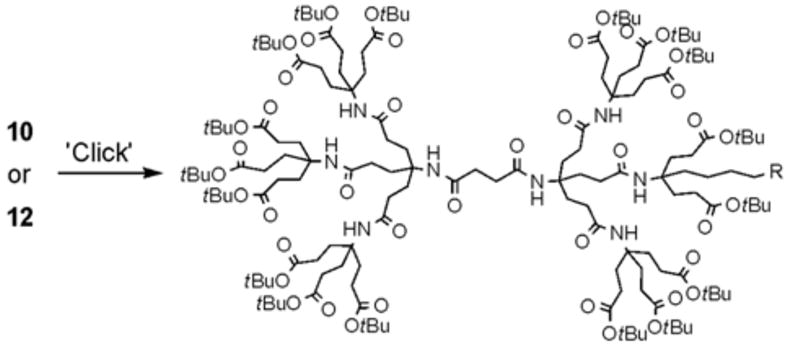
The establishment of an easy protocol for the monofunctionalization is key to our research strategy. Therefore, we investigated the functionalization of dendrimers 10 and 12 using 1,3 dipolar cycloadditions between an azide and an alkyne moiety. Unlike the typical 1,3 dipolar cycloaddition conditions at moderate temperature, our preliminary study using acetylene dendrimer 10 and benzyl azide under the ‘typical’ reaction conditions showed incomplete conversions and temperatures of 80 °C for 20 h were needed to obtain quantitative yields (Scheme 4). To reduce the reaction time and to have less harsh reaction conditions, we investigated the monomodal microwave reactor assisted 1,3 dipolar cycloaddition-based functionalization of our dendrimers. 1,3 Dipolar cycloadditions can be assisted by microwave irradiation to reduce the reaction time significantly and to provide near perfect regioselectivity.13 The microwave assisted 1,3 dipolar cycloadditon has been utilized for biological systems including the attachment of peptides onto a dendritic molecules14 and the conjugation of oligonucleotides and carbohydrates.15
Scheme 4.
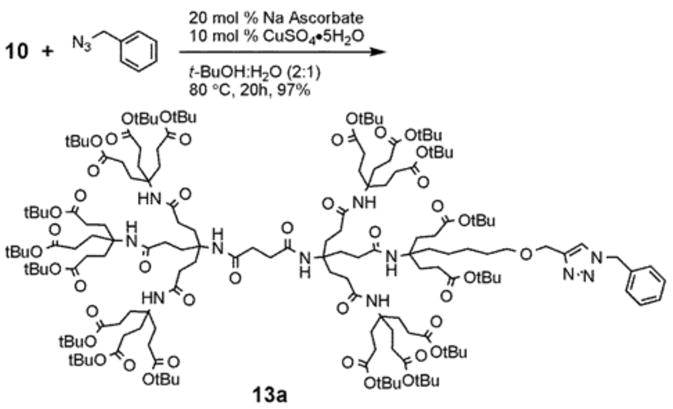
1,3 Dipolar cycloaddition functionalization using conventional heating conditions.
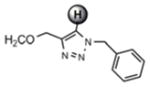
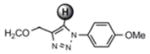
For the microwave experiments, 10 was treated with benzyl azide16 under typical 1,3 dipolar cycloaddition conditions using Na ascorbate and CuSO4·5H2O in a 1:1 mixture of t-BuOH and H2O in a sealed glass vial. The microwave reactor was set-up using the power-time control method at 100 W irradiation power and a shut-off temperature of 100 °C.14 The reaction was terminated after 10 minutes to afford the desired triazole product 13a in 98% isolated yields. While the yields are similar to the ones described above using conventional heating methods, the microwave irradiation significantly cuts down the reaction time from hours to minutes. Table 1 summarizes the microwave assisted 1,3 dipolar cycloaddition reactions that were carried out on dendrimers 10 and 12. For all reactions studied under microwave conditions, we obtained excellent isolated yields (95–98%). Furthermore, when comparing the alkyne functionalized dendrimer (10) with the azide functionalized one (12), we did not detect any significant reactivity differences.
Table 1.
Microwave assisted 1,3 dipolar cycloadditions of 10 and 12.a
 | |||
|---|---|---|---|
| dendrimer | reactant | product (R = ) | 1H NMR (δ)b/yield |
| 10 |
13a |
|
7.62 ppm
98% |
| 10 |
13b |
|
8.07 ppm
95% |
| 12 |
13c |

|
7.93 ppm
98% |
| 12 |
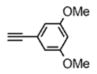
13d |
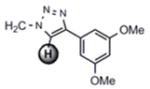
|
7.87 ppm
97% |
Reaction conditions: t-BuOH:H2O (1:1), 10 mol % Na ascorbate, 5 mol %CuSO4·5H2O. Microwave setting: Power-time control method, 100W, 10 min, Pmax= 65 psi, and Tmax= 100°C.
Chemical shift for the newly formed triazole protons.
In conclusion, we have prepared monofunctionalized dendrons and dendrimers containing either a single alkyne or azide moiety. The resulting dendrimers containing a single chemical handle can be functionalized via 1,3 dipolar cycloadditon in outstanding yields. Along with the attachment of biological moieties on a single chemical handle, further functionalizations are available for these dendrimers via a deprotection of the tert-butyl esters on the periphery and subsequent reaction using the terminal carboxylic acids. The employed convergent approach potentially allows for the introduction of a second orthogonal functionality on the surface of the dendrimers. Such studies are currently under investigation.
Supplementary Material
Experimental details and characterization data (1H and 13C NMR, MS, and EA) of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We gratefully thank the National Institute of Health (NIH P20-GM072021) for financial support of this research. MW acknowledges a 3M Untenured Faculty Award, a DuPont Young Professor Award, a Camille Dreyfus Teacher/Scholar Award and an Alfred P. Sloan Fellowship.
References
- 1.(a) Fischer M, Vögtle F. Angew Chem Int Ed. 1999;38:884–905. doi: 10.1002/(SICI)1521-3773(19990401)38:7<884::AID-ANIE884>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]; (b) Bosman AW, Janssen HM, Meijer EW. Chem Rev. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]; (c) Newkome GR, Moorefield CN, Vögtle F. Dendrimers and Dendrons: Concepts, Syntheses, Applications. Wiley; New York: 2001. [Google Scholar]; (d) Helms B, Meijer EW. Science. 2006;313:929–923. doi: 10.1126/science.1130639. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cloninger MJ. Curr Opin Biotechnol. 2002;6:742–748. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]; (b) Roberts JC, Bhalgat MK, Zera RT. J Biomed Mat Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.(a) Dykes GM. J Chem Tech Biotech. 2001;76:903–918. [Google Scholar]; (b) Esfand R, Tomalia DA. Drug Dis Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 4.(a) Balogh L, Tomalia DA. J Am Chem Soc. 1998;120:7355–7356. [Google Scholar]; (b) Crooks RM, Zhao MQ, Sun L, Chechik V, Yeung LK. Acc Chem Res. 2001;34:181–190. doi: 10.1021/ar000110a. [DOI] [PubMed] [Google Scholar]
- 5.(a) Bielinska A, Eichman JD, Lee I, Baker JR, Jr, Balogh L. J Nanopart Res. 2002;4:395–403. [Google Scholar]; (b) Zheng J, Dickson RM. J Am Chem Soc. 2002;124:13982–13983. doi: 10.1021/ja028282l. [DOI] [PubMed] [Google Scholar]; (c) Huang K, Cohen MJ, Croce T, Hamilton S, Evans BL, Voss B, Hamm HE, Harth E. Bioconjugate Chem. 2007;18:403–409. doi: 10.1021/bc060287a. [DOI] [PubMed] [Google Scholar]
- 6.For examples of multiple functionalities on the periphery of dendrimers, see: Hawker CJ, Fréchet JMJ. Macromolecules. 1990;23:4726–4729.Bo Z, Schäfer A, Franke P, Schlüter AD. Org Lett. 2000;2:1645–1648. doi: 10.1021/ol005972q.Zhang W, Nowlan DT, III, Thomson LM, Lackowski WM, Simanek EE. J Am Chem Soc. 2001;123:8914–8922. doi: 10.1021/ja0041369.Ganesh RN, Shraberg J, Sheridan PG, Thayumanavan S. Tetrahedron Lett. 2002;43:7217–7220.Mihov G, Scheppelmann I, Müllen K. J Org Chem. 2004;69:8029–8037. doi: 10.1021/jo048998u.
- 7.(a) Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (b) Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Fréchet JMJ, Sharpless KB, Fokin VV. Angew Chem Int Ed. 2004;43:3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]; (d) Hawker CJ, Wooley KL. Science. 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- 8.For some recent examples of the use of 1,3 dipolar cycloaddition in polymer chemistry see: Helms B, Mynar JL, Hawker CJ, Fréchet JMJ. J Am Chem Soc. 2004;126:15020–15021. doi: 10.1021/ja044744e.Binder WH, Sachsenhofer R. Macromol Rapid Commun. 2007;28:15–54.Hoogenboom R, Moore BC, Schubert US. Chem Commun. 2006;38:4010–4012. doi: 10.1039/b608313g.Wang XY, Kimyonok A, Weck M. Chem Commun. 2006;37:3933–3935. doi: 10.1039/b609382e.Quemener D, Davis TP, Barner-Kowollik C, Stenzel MH. Chem Commun. 2006;48:5051–5053. doi: 10.1039/b611224b.Opsteen JA, Van Hest JCM. Chem Commun. 2005;1:57–59. doi: 10.1039/b412930j.
- 9.For the employment of 1,3 dipolar cycloaddition in dendrimer chemistry see: Gopin A, Ebner S, Attali B, Shabat D. Bioconjugate Chem. 2006;17:1432–1440. doi: 10.1021/bc060180n.Lee JW, Kim JH, Kim B. Tetrahedron Lett. 2006;47:2683–2686.Wu P, Malkoch M, Hunt JN, Vestberg R, Kaltgrad E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. Chem Commun. 2005;46:5775–5777. doi: 10.1039/b512021g.Rijkers DTS, Van Esse GW, Merkx R, Brouwer AJ, Jacobs HJF, Pieters RJ, Liskamp RMJ. Chem Commun. 2005;36:4581–4583. doi: 10.1039/b507975f.Joralemon MJ, O’Reily RK, Matson JB, Nugent AK, Hawker CJ, Wooley KL. Macromolecules. 2005;38:5436–5443.
- 10.Newkome GR, Kim HJ, Moorefield CN, Maddi H, Yoo KS. Macromolecules. 2003;36:4345–4354. [Google Scholar]
- 11.(a) Newkome GR, Behera RK, Moorefield CN, Baker GR. J Org Chem. 1991;56:7162–7167. [Google Scholar]; (b) Newkome GR, Yoo KS, Moorefield CN. Designed Monomers Polym. 2002;5:67–77. [Google Scholar]
- 12.Ballini R, Petrini M, Rosini G. Tetrahedron. 1990;46:7531–7538. [Google Scholar]
- 13.Appukkuttan P, Dehaen W, Fokin VV, Van der Eycken E. Org Lett. 2004;6:4223–4225. doi: 10.1021/ol048341v. [DOI] [PubMed] [Google Scholar]
- 14.Rijkers DTS, van Esse GW, Merkx R, Brouwer AJ, Jacobs HJF, Pieters RJ, Liskamp RMJ. Chem Commun. 2005:4581–4583. doi: 10.1039/b507975f. [DOI] [PubMed] [Google Scholar]
- 15.Bouillon C, Meyer A, Vidal S, Jochum A, Chevolot Y, Cloarec JJ, Praly JP, Vasseur JJ, Morvan F. J Org Chem. 2006;71:4700–4702. doi: 10.1021/jo060572n. [DOI] [PubMed] [Google Scholar]
- 16.Demko ZP, Sharpless KB. Angew Chem Int Ed. 2002;41:2110–2113. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details and characterization data (1H and 13C NMR, MS, and EA) of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.


