Arterial calcification increasingly afflicts our aging populace(1). Approximately 2% of individuals over age 65 will require aortic valve replacement (AVR) for calcific aortic stenosis(1). Based upon recent epidemiologic studies (2), the increasing prevalence of metabolic syndrome and type II diabetes (T2DM) will further increase the need for AVR – unless strategies are identified and implemented that prevent or reverse valve calcification. Similar concerns exist for two other types of vascular mineral deposition, atherosclerotic intimal calcification and medial artery calcification(3). Medial calcification is a strong predictor of lower extremity amputation in T2DM(4), a debilitating and costly outcome. Perturbed Windkessel physiology and altered vascular autonomic responses lead to tissue ischemia(5). Microcalcifications of cholesterol-laden or fibrous components of coronary atherosclerotic plaques attend outward vascular remodeling(6) -- harbingers of acute coronary syndrome(7). A better understanding of arterial calcification and vascular mineral metabolism is needed. Once considered only a passive process of dead and dying cells, data from laboratories world-wide have shown that vascular calcification is an actively regulated form of tissue biomineralization(3). In response to metabolic, mechanical, and inflammatory insults, vascular mesenchymal cells elaborate matrix vesicles and gene regulatory programs that drive (a) osteogenic vascular matrix remodeling(8); and (b) locally neutralize paracrine and systemic inhibitors of calcium deposition (9).
In this issue of the Journal, Miller, Heistad, and colleagues (10)present an enlightening study that not only reveals the mechanistic underpinnings of human aortic valve calcification, but also highlights the critical role of reactive oxygen species (ROS) to the pathobiology of most forms of arterial mineralization. Using dihydroethidium (DHE) staining and lucigenin chemiluminescence, the authors identified increased superoxide levels in stenotic calcified valves vs. normal human heart valves. DHE staining spatially resolved a gradient of oxidative stress within calcifying aortic valves, with highest levels localizing to regions possessing extensive calcium deposition(10). DCF (dichlorodihydrofluorescein) staining for hydrogen peroxide – the more durable ROS product of dismutation that propagates intracellular signals and iron-catalyzed oxidative damage (Figure 1) -- is also increased in regions of valve calcification, notably at the leaflet base(10). This was not due to increased superoxide dismutase (SOD) expression, since SOD isoforms and activities were down-regulated. More importantly, for reasons to be discussed, Catalase expression was reduced in both calcified and non-calcified segments of diseased valves as compared to normal valves. Thus, increases in ROS “tone” in aortic valves undergoing calcification are accompanied by reductions in defenses that remove several reactive oxygen species(10) -- including the second messenger, hydrogen peroxide(11).
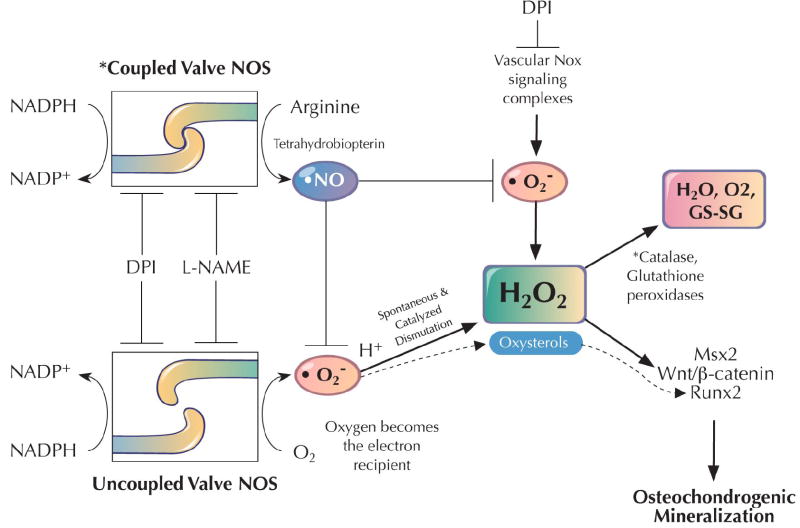
Figure 1. Working model of hydrogen peroxide actions during vascular calcification.
In response to uncoupled NOS and/or vascular Nox activity, arterial peroxide levels are increased in the setting of impaired peroxidase defenses that dissipate H2O2 signals. In the calcifying aortic valve, uncoupling of NOS activity prominently contributes along with reductions in valve catalase activity(10). H2O2 upregulates Msx2 (21), Runx2/Cbfa1(24), and Wnt/β-catenin cascades (23) necessary for osteogenic differentiation of multipotent vascular osteoprogenitors(3,16). GS-SG, oxidized glutathione.
NADPH Oxidases: The Road Not Taken
NADPH oxidase / Nox activities(12) figure prominently in arterial oxidative stress . arising from non-laminar flow, inflammatory cytokine signaling, and activation of the renin-angiotensin-aldosterone system (13,14). Nox1 and Nox2 play critical roles in the aortic remodeling entrained to angiotensin (13,14). Thus, Miller evaluated whether Nox subunits were increased at venues of aortic valve calcification and oxidative stress (10). Surprisingly, Nox isoforms were uniformly decreased in calcifying valve segments, and no significant differences in Nox-dependent superoxide generation were measured between normal and diseased valves (10). This was completely unexpected because of the contributions of Nox signaling to atherosclerosis and vascular remodeling(11),. DPI (diphenyliodonium) -- an inhibitor of flavoenzymes such as Nox, xanthine oxidase, and nitric oxidase synthase (NOS)(12) -- did inhibit superoxide elaborated by calcifying valvular cells, confirming an enzymatic contribution to the generation of valve ROS.
When uncoupled by tetrahydrobiopterin deficiency or inflammation that precludes homodimer formation, NOS monomers utilize molecular oxygen -- rather than arginine – as the terminal electron recipient in the NOS NADPH/flavin/iron relay(15) (Figure 1). Therefore, the authors astutely examined the impact of selective NOS inhibition on valve superoxide, implementing the antagonistic arginine analog, L-NAME. L-NAME reduced superoxide production, indicating the contribution of NOS uncoupling to calcified aortic valve ROS generation(10). Had valvular NOS been in coupled, L-NAME treatment would have increased superoxide accrual – since NOS-dependent nitric oxide production scavanges superoxide via peroxynitrite formation (15) (Figure 1). Thus, Miller et al. demonstrate that calcifying aortic valves generate a surfeit of superoxide and peroxide via uncoupled NOS activity in the setting of impaired antioxidant defenses – viz., valvular catalase deficiency and reduced NO production(10) (Figure 1, asterisks).
Vascular Oxidative Stress Presages Osteochondrogenic Programming
The authors then s related spatial patterns of aortic valve oxidative stress to the elaboration of osteochondrogenic transcription factors known to program biomineralization(16). Runx2/Cbfa1, Msx1, and Msx2 play critical roles in osteogenic mineralization (17). Runx2/Cbfa1 and Msx2 had been previously identified in calcifying human arteries(18); moreover, in a model of diabetic aortic calcification, Msx2 participates in a signaling relay that entrains osteogenic Wnt/β-catenin signaling to vascular inflammation(19,20). Miller identified that Runx2/Cbfa1 and Msx2 were indeed expressed in calcifying human aortic valves(10), confirming the contribution of active osteochondrogenic regulatory programs to valve calcium accrual(1). Once again, however, another surprise emerged. While the expression of Msx2 was tightly entrained to regions of valve biomineralization, Runx2/Cbfa1 expression was visualized most robustly in adjacent diseased valve segments –confirmed by RT-qPCR analysis(10) The segregation of Msx2 and Runx2/Cbfa1 expression into distinct domains within diseased valves may reflect the actions of the paracrine Wnt signaling milieu that programs osteogenesis (17). Via the cell-surface receptors LRP5 and LPR6, canonical Wnt ligands induce dimerization with co-receptors that activate nuclear β-catenin signals necessary for osteogenic differentiation (reviewed in (17)). Conversely, these pathways are inhibited by antagonistic ligands such as Dkk1(17,20). Since Msx2-positive cells elaborate canonical Wnt ligands (Wnt3a and Wnt7a) -- but express very little if any Dkk1(20) --- cells in the adjacent vicinity may upregulate Runx2/Cbfa1 expression, a target of Wnt signaling in bone(17). However, the relationship of nuclear β-catenin accumulation to the spatial patterns of Msx2 and Runx2/Cbfa1 expression in calcifying valves has yet to be assessed. Of note, Rajamannan has clearly shown that Wnt3a, LRP5, and β-catenin are upregulated in calcifying human aortic valves as compared to non-calcifying specimens (1)
Peroxide Paves The Path Of Vascular Osteogenesis
Why is this study so significant? In addition to identifying that it is NOS uncoupling –not Nox activation – that generates ROS in calcifying human aortic valves, the authors demonstrate increased accumulation of hydrogen peroxide (H2O2) in calcifying valve segments(10). H2O2 is a pro-inflammatory second messenger(11). In preclinical models of diabetic arterial diseases, H2O2 is initially generated via TNFα-dependent Nox activation -- upstream of arterial Msx2-Wnt expression(21,22). Furthermore, at low levels, H2O2 promotes nuclear β-catenin signaling by inhibiting nucleoredoxin(23). Recently, Chen has shown that H2O2 can also upregulate Runx2/Cbfa1 expression and promote osteogenic mineralization of vascular smooth muscle (24). Thus, Miller’s insightful molecular study of human aortic valve calcification (10) converges with accumulating pre-clinical data to highlight the fundamental contributions of peroxide signaling to vascular calcification (Figure 1). Aortic valve H2O2 accumulates in part due to valvular catalase deficiency(10). Since perturbed expression of Gpx1 is associated with increased coronary calcification in T2DM(25), futures studies may address whether glutathione peroxidases also participate in maintaining aortic valve longevity in T2DM.
The Opportunities: Avoiding Loss In Translation
Certainly, much more remains to be done to translate these seminal observations into clinical practice. The specific NOS isoforms that contribute to aortic valve disease with aging remain to evaluated; eNOS plays an important role in valve morphogenesis, and deficiency predisposes to biscuspid valve calcification (1,16). The reasons for aortic valve NOS uncoupling remain to be determined(15) – and may differ during disease initiation and progression. In addition to tetrahydrobiopterin deficiency, oxidative stress itself can uncouple NOS(15). This has clinical implications, since once vascular mineral is deposited, it induces further inflammation and oxidative stresses (26). Pro-active nutritional and pharmacologic strategies that reduce NOS uncoupling and enhance valve peroxidase activities may help prevent aortic valve calcification (Figure 1) (1). The mechanisms of acquired catalase deficiency -- and relative contributions of Catalase vs. Gpx isoforms to aortic valve peroxide tone -- remain to be determined (Figure 1). Interactions between ROS generation and the neoangiogenesis necessary for true “ossification” – seen in approximately 15% of calcified aortic valve specimens(27) – remain to be established. The absence of Nox subunit induction with disease progression does not mean that Nox signaling is unimportant for healthy aortic valves. Nox4 is critical to maintenance of the vascular myofibroblast phenotype(28). Thus, down-regulation of valvular Nox4 (10) may permit osteogenic “trans-differentiation” of valve myofibroblasts. Oxysterols, bioactive components of oxidized LDL, also upregulate Runx2-dependent transcription(29). Thus, deficiencies in the enzymes that reduce lipoprotein oxidation may also contribute. Finally, once initiated, a substantial portion of aortic valve calcium accrual occurs via amorphous epitaxial mineral deposition that is independent of osteogenic cells(27) -- and may be enhanced by cholesterol (30) . This represents a failure of local and circulating tissue mineralization inhibitors such as fetuin(9) and osteopontin(31). Although osteopontin is increased in regions of calcification and inhibits mineral deposition, its bioactivity is regulated by processing that generates pro-inflammatory fragments(31). The impact of ROS on fetuin and osteopontin functions has not been studied. All in all, the study of Miller et al (10) affords us a significantly improved understanding of aortic vascular calcification. It initiates a new era of investigation into the biology and pharmacology of calcific aortic stenosis, offering new hope for the prevention and medical treatment of an otherwise burgeoning clinical need (1,16).
Acknowledgments
Supported by NIH grants HL69229, HL81138, HL88651, AR43731, and the Barnes-Jewish Hospital Foundation.
Footnotes
Disclosure: Dr. Towler has served as a paid consultant on musculoskeletal disease therapeutics for Wyeth, GlaxoSmithKline, and Eli Lilly. He receives funding for his research from the National Institutes of Health, and from the Barnes-Jewish Hospital Foundation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Rajamannan NM, Bonow RO, Rahimtoola SH. Calcific aortic stenosis: an update. Nat Clin Pract Cardiovasc Med. 2007;4:254–62. doi: 10.1038/ncpcardio0827. [DOI] [PubMed] [Google Scholar]
- 2.Katz R, Wong ND, Kronmal R, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–9. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 3.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 4.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 5.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–30. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 6.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 7.Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 8.Aikawa E, Nahrendorf M, Figueiredo JL, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–50. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–59. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 10.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of anti-oxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008 doi: 10.1016/j.jacc.2008.05.043. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–89. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Ormsby A, Oja-Tebbe N, Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res. 2004;95:587–94. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 14.Dikalova A, Clempus R, Lassegue B, et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–76. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 15.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–7. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–8. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 17.Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41–9. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–94. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 19.Al-Aly Z, Shao JS, Lai CF, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–96. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 20.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao JS, Aly ZA, Lai CF, et al. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 22.Lai-Huang CF, Shal JS, Al-Aly Z, Cheng SL, Towler DA. TNF-alpha Dependent Redox Signals Upregulate Msx2 Gene Transcription via Stress Activated Protein Kinases in Arterial Myofibroblasts. J Bone Miner Res. 2007;22:Abstract T165. [Google Scholar]
- 23.Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–8. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 24.Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor runx-2 by akt signaling. J Biol Chem. 2008 doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemoto M, Nishimura R, Sasaki T, et al. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc Diabetol. 2007;6:23. doi: 10.1186/1475-2840-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanahan CM. Inflammation ushers in calcification: a cycle of damage and protection? Circulation. 2007;116:2782–5. doi: 10.1161/CIRCULATIONAHA.107.749655. [DOI] [PubMed] [Google Scholar]
- 27.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 28.Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–7. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 29.Richardson JA, Amantea CM, Kianmahd B, et al. Oxysterol-induced osteoblastic differentiation of pluripotent mesenchymal cells is mediated through a PKC- and PKA-dependent pathway. J Cell Biochem. 2007;100:1131–45. doi: 10.1002/jcb.21112. [DOI] [PubMed] [Google Scholar]
- 30.Laird DF, Mucalo MR, Yokogawa Y. Growth of calcium hydroxyapatite (Ca-HAp) on cholesterol and cholestanol crystals from a simulated body fluid: A possible insight into the pathological calcifications associated with atherosclerosis. J Colloid Interface Sci. 2006;295:348–63. doi: 10.1016/j.jcis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–9. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]