Abstract
X-linked Emery-Dreifuss muscular dystrophy (X-EDMD) is inherited through mutations in EMD, which encodes a nuclear membrane protein named emerin. Emerin is expressed in most cells, but EDMD strikes specific tissues. This review summarizes growing evidence that emerin has roles in both tissue-specific gene regulation and the mechanical integrity of the nucleus, and discusses how these roles might impact EDMD.
Keywords: laminopathy, nuclear envelope, lamin, Emery-Dreifuss muscular dystrophy
Introduction
Emery-Dreifuss muscular dystrophy (EDMD) is inherited through mutations in either of two genes: LMNA, encoding A-type lamins, and EMD, which encodes a nuclear membrane protein named emerin (Emery, 2000). EDMD is characterized by progressive skeletal muscle weakening, contractures of major tendons and potentially fatal cardiac conduction defects (Cohen et al., 2001; Emery, 2000). Emerin is an integral protein of the nuclear inner membrane (Nagano et al., 1996) that is expressed in nearly all human cells (one exception being non-myocytes of the heart; Manilal et al., 1996). Emerin belongs to the ‘LEM-domain’ family of nuclear proteins, defined by a ~40-residue domain (the ‘LEM domain’). Other members include LAP2α, LAP2β, MAN1 (Cohen et al., 2001; Dechat et al., 2000; Lin et al., 2000) and several uncharacterized human genes including lem-2 (Lee and Wilson, 2004), which was identified as NET-25 in a proteomic analysis of nuclear envelope transmembrane (NET) proteins (Schirmer et al., 2003) and is conserved in C. elegans (Lee et al., 2000). A major shared function of all studied LEM-domain proteins is their binding (via the LEM domain) to a chromatin protein named Barrier-to-Autointegration Factor (BAF; Margalit et al., 2005; Segura-Totten and Wilson, 2004). BAF is a small, highly conserved essential protein with direct roles in higher-order chromatin structure (Segura-Totten et al., 2002), nuclear assembly (Haraguchi et al., 2001; Segura-Totten et al., 2002), and gene regulation (Holaska et al., 2003; Margalit et al., 2005; Wang et al., 2002). Emerin also binds lamins, which anchor emerin at the nuclear inner membrane (Burke and Stewart, 2002; Holaska et al., 2002). Together, emerin and lamin A form stable ternary complexes with other binding partners in vitro (Holaska et al., 2003), with the potential to form a variety of oligomeric protein complexes (Bengtsson and Wilson, 2004).
To explain the tissue specificity of EDMD, emerin was proposed to have roles in tissue-specific gene expression, signal transduction or mechanical stability (Bonne et al., 2003; Cohen et al., 2001; Ostlund and Worman, 2003). However it was soon apparent that no single model would suffice. Mechanical models failed to explain the cardiac conduction phenotype of EDMD, but were consistent with structural defects (aberrant shape and nuclear envelope herniations) seen in nuclei from EDMD patients (Fidzianska and Hausmanowa-Petrusewicz, 2003) and in a subset of patients with other diseases (laminopathies) linked to mutations in LMNA (Holaska et al., 2002; Ostlund and Worman, 2003; Somech et al., 2005). Similarly, it was not obvious how gene regulation or signaling roles for emerin could explain defects in nuclear architecture. This conundrum has been solved by growing evidence that emerin has multiple roles, each of which is likely to be important for the EDMD disease mechanism. This review will focus on emerin’s (a) architectural, (b) gene regulatory and (c) nuclear assembly roles.
Evidence that emerin has architectural roles
Emerin and lamins
Emerin binds directly to both A- and B-type lamins in vitro (Clements et al., 2000; Lee et al., 2001) and co-localizes with lamins in vivo (Goldman et al., 2005; Manilal et al., 1998). Lamins are type V intermediate filament proteins that stabilize the nuclear envelope (Goldman et al., 2005), and anchor nuclear membrane proteins including emerin. A-type lamins are specifically required for emerin localization, since cells lacking A-type lamins mislocalize emerin to the endoplasmic reticulum (ER; Muchir et al., 2003; Sullivan et al., 1999). Interestingly, in skin fibroblasts from emerin-null patients, lamins A, C and B2 are more soluble (Markiewicz et al., 2002), suggesting disrupted lamina architecture. Furthermore, lamin C grossly mislocalizes to the nuclear interior, while lamin A remains at the nuclear envelope (Markiewicz et al., 2002). It is not known why loss of emerin selectively disrupts lamin C localization, but these results suggest emerin contributes positively to the stability of the lamin network. Muscle cells from emerin-null patients also have aberrant nuclear envelope architecture and heterochromatin organization, as seen by electron microscopy (Fidzianska and Hausmanowa-Petrusewicz, 2003). Muscle nuclei from these patients also had excess heterochromatin, and were also highly fragile when isolated (Fidzianska and Hausmanowa-Petrusewicz, 2003), consistent with mechanical weakness.
Emerin and nuclear actin
Actin is present in the nucleus (Pederson and Aebi, 2002) and is likely to be important for nuclear architecture. Emerin binds directly to globular (G-) actin (Fairley et al., 1999; Lattanzi et al., 2003). More significantly, emerin also binds filamentous (F-) actin with ~480 nM affinity and stabilizes F-actin by capping the pointed end (Holaska et al., 2004). A missense mutation in emerin (Q133H) that is sufficient to cause EDMD specifically disrupts binding to F-actin (Holaska et al., 2004), but has no effect on emerin’s binding to transcription regulators germ cell-less (GCL), Bcl-2 associated transcription factor (Btf) or YT521-B (Haraguchi et al., 2004; Holaska et al., 2003; Wilkinson et al., 2003). Thus loss of emerin binding to F-actin is one potential molecular mechanism for EDMD. Other ‘nucleoskeletal’ proteins including αII-spectrin also co-purified with emerin by affinity chromatography, suggesting emerin might anchor an actin-spectrin network at the inner nuclear membrane (Holaska et al., 2004). Since lamins also bind both emerin (discussed above) and actin (Lattanzi et al., 2003), this proposed emerin-actin-spectrin network might stabilize the lamina network. Loss of emerin might cause EDMD by disrupting an actin-based lamin-reinforcing system needed for nuclear integrity. This system might be particularly important for tissues that experience high mechanical loads, such as muscle and tendons, which are affected by EDMD.
Additional evidence supports the interaction of emerin and actin. First, emerin forms a complex with lamin A and actin (Lattanzi et al., 2003). Second, actin localizes to the nuclear envelope under certain fixation conditions and co-immunoprecipitates with emerin from C2C12 myotubes (Maraldi et al., 2004). Maraldi et al. (2004) hypothesized that muscle differentiation programs regulate emerin binding to actin, since emerin bound actin in myotubes but not myoblasts. When C2C12 myotubes were treated with okadaic acid, an inhibitor of Protein Phosphatase 1, less actin co-immunoprecipitated with emerin (Maraldi et al., 2004), suggesting that phosphorylation inhibits the emerin-actin interaction, either directly or indirectly.
Emerin and nesprins
Architectural roles for emerin might also involve a new family of proteins known as nesprins, which have spectrin repeat (SR) domains. Nesprins are encoded by two genes, now known as nesprin-1 and nesprin-2 (previous names include Myne1, Syne1, Enaptin, and MSP300; Warren et al., 2005). Each nesprin gene is alternatively spliced and produces at least 7 different isoforms in vivo (Warren et al., 2005). Besides their SR domains, many nesprins also have a C-terminal ‘klarsicht-like’ domain, which is required for nuclear envelope localization (Warren et al., 2005). The largest nesprin isoforms also contain calponin homology domains, which bind actin directly (Warren et al., 2005). The nesprin-1α isoform is an integral inner nuclear membrane protein that is preferentially expressed in muscle (Mislow et al., 2002b) and co-localizes with emerin at the nuclear envelope (Zhang et al., 2001). Nesprin-1α binds emerin tightly, with an affinity of 4 nM in vitro (Mislow et al., 2002a). Nesprin-1α also binds lamin A (Mislow et al., 2002a), suggesting nesprin-1α might co-anchor both emerin and lamin A. Nesprin-2α and nesprin-2β also bind directly to emerin and lamin A via their C-terminus (Libotte et al., 2005; Zhang et al., 2005). Interestingly, Lmna-null cells mislocalize nesprin-1, nesprin-2 and emerin to the ER (Libotte et al., 2005; Zhang et al., 2005). Lamin C is sufficient for nesprin-2 localization, since nesprin-2 is nuclear envelope-localized in cells with lamin C as their only A-type lamin (Libotte et al., 2005). RNAi-mediated downregulation of nesprin-2 mislocalizes emerin to the ER, but has no effect on lamin A localization (Libotte et al., 2005). Downregulation of emerin has no effect on nesprin-2 localization (Libotte et al., 2005). Thus lamins and nesprins are each necessary for emerin localization at the nuclear envelope. Interestingly, the Q133H and P183H mutations in emerin, which cause EDMD, disrupt binding to nesprins, but have little affect on emerin’s transcriptional regulatory partners (Holaska and Wilson, unpublished observation).
Roles for emerin in gene regulation
Direct evidence that emerin regulates tissue-specific gene expression is still lacking. However, indirect evidence supports this idea. Emerin binds directly to at least two transcription repressors, GCL (Holaska et al., 2003) and Btf (Haraguchi et al., 2004), and splicing factor YT521-B (Wilkinson et al., 2003). Emerin is closely related to LAP2β, a LEM-domain protein that also binds GCL and represses gene expression in vivo (Nili et al., 2001). Furthermore, changes in the expression levels of approximately 60 genes (out of 2400 total) were seen in cultured fibroblasts derived from X-EDMD patients (Tsukahara et al., 2002), and for 28 of these genes, normal expression was restored by supplying wildtype emerin. These results, while limited in scale, strongly suggest that emerin influences gene expression, most likely through binding to gene-regulatory partners. However, none of these genes are expressed only in EDMD target tissues, and therefore are most likely not the ‘smoking gun’. Known partners are discussed below.
GCL
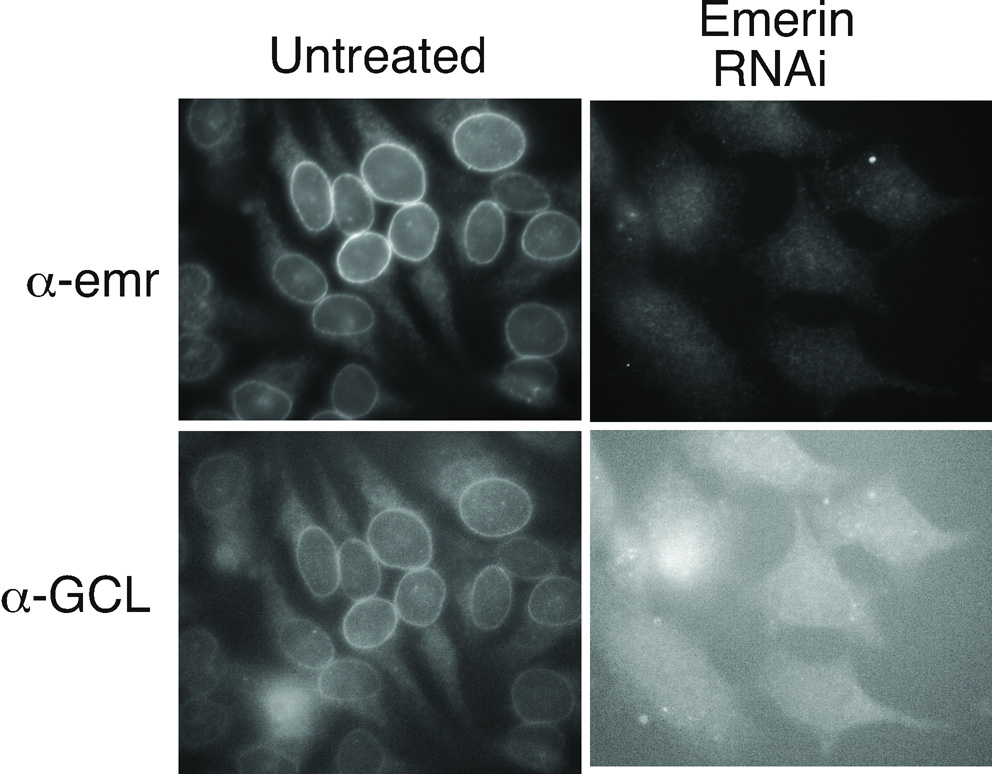
GCL represses transcription by binding the DP3 subunit of E2F-DP3 heterodimers, which are transcriptional activators (de la Luna et al., 1999). LAP2β, which is 41% similar to emerin, represses E2F-DP reporter gene activity in vivo; furthermore, transient co-expression of both LAP2β and GCL represses transcription as effectively as Rb in vivo (Nili et al., 2001). Emerin binds GCL with 30 nM affinity, and mapping studies identified two binding domains in emerin, named repressor-binding domains (RBD) 1 and 2 (Holaska et al., 2003), which are also required for emerin to bind other gene-regulatory partners, including Btf and YT521-B (Haraguchi et al., 2004; Wilkinson et al., 2003). Further studies showed that BAF competes with GCL for binding to emerin, forming either of two distinct complexes comprising BAF-lamin A-emerin or GCL-lamin A-emerin, (Holaska et al., 2003). Interestingly, RNAi-mediated downregulation of emerin in HeLa cells causes GCL to mislocalize from the nuclear envelope and nucleoplasm to the cytoplasm (Figure 1), concomitant with increased E2F-DP-mediated gene expression. This suggests that emerin is required for GCL to function as a repressor.
Figure 1. Emerin-dependent nuclear envelope localization of GCL.
HeLa cells were treated with either siRNA specific to emerin (5’-CAUCCCGCACGGGCCUGUAdTdT-3’ and 5’-UACAGGCCCGUGCGGGAUGdTdT-3’) or untreated (oligofectamine alone), per manufacturer’s instructions (Dharmacon, Inc.). After 48 h the cells were fixed with formaldehyde and stained with antibodies against emerin (α-Emr, serum 2999) and GCL (α-GCL, serum 4439).
Btf
Emerin also binds the pro-apoptotic transcription repressor Btf (Haraguchi et al., 2004). In vitro binding assays showed that emerin binds residues 377–646 of Btf with 100 nM affinity (Haraguchi et al., 2004). Importantly, Btf relocalizes from the nuclear interior to the nuclear envelope upon induction of apoptosis (Haraguchi et al., 2004), suggesting that Btf may require emerin to signal apoptosis within the nucleus.
YT521-B
YT521-B influences splice-site selection in vitro and in vivo (Hartmann et al., 1999), and was identified as an emerin-binding protein in a yeast two-hybrid screen (Wilkinson et al., 2003). The C-terminus of YT521-B is sufficient to bind emerin in vitro and influence splice-site selection in vivo (Wilkinson et al., 2003), suggesting that emerin might influence mRNA splicing.
Undiscovered emerin-binding proteins
GCL, Btf and YT521-B cannot be the only transcription regulators that bind emerin. Additional gene-regulatory partners remain to be discovered, and are likely to compete for binding to the RBD-1 and RBD-2 domains in emerin. Our challenge is to find the gene-regulatory partners directly relevant to the heart, muscle and tendon pathology of EDMD patients.
Further support for gene-regulatory roles for emerin comes from an unlikely source: another LEM-domain protein named MAN1. MAN1 regulates signal transduction by binding directly to SMADs, which mediate TGF-β signaling during development and in specialized tissues (Gruenbaum et al., 2005; Lin et al., 2005; Osada et al., 2003; Raju et al., 2003). MAN1 also binds directly to emerin (Mansharamani and Wilson, 2005). Interestingly, emerin binding to MAN1 is reduced by the emerin Q133H mutation (which also disrupts binding to nesprin-1α) that causes EDMD (Mansharamani and Wilson, 2005). Thus we hypothesize that emerin might influence TGF-β signaling in tissues affected by EDMD.
Evidence from C. elegans shows that emerin also has significant functional overlap with lem-2 (also known as Ce-MAN1; Liu et al., 2003). When Ce-lem-2 was downregulated 90% using RNAi, approximately 15% of embryos died, whereas complete loss of emerin by RNAi has no phenotype (Liu et al., 2003). When both proteins were downregulated, 90% of embryos died by the 100-cell stage (Liu et al., 2003), demonstrating that Ce-lem-2 and Ce-emerin have overlapping functions in vivo. This predicts that human emerin and human lem-2 also overlap in function. Because lem-2 is closely related to MAN1, but is not predicted to bind SMADs, we hypothesize that lem-2 (and hence emerin) might influence other unknown signaling pathways.
Roles for emerin in nuclear assembly
BAF directly binds emerin in vitro (Holaska et al., 2003; Lee et al., 2001) and is proposed to regulate emerin-mediated gene expression by competing with other transcription factors (Holaska et al., 2003). However, until recently it was not known whether BAF directly binds emerin at the nuclear envelope in vivo. Fluorescence resonance energy transfer (FRET) experiments showed that CFP-BAF binds YFP-emerin at the nuclear envelope in living cells (Shimi et al., 2004). Fluorescence recovery after photobleaching (FRAP) experiments further showed that BAF is highly mobile (t1/2 = 270 ms) whereas emerin, as expected, is relatively immobile (t1/2= 62 s; Shimi et al., 2004). The mobility of BAF during interphase contrasts with mitosis: in telophase BAF and emerin are stably recruited to the ‘core’ region of chromosomes for 4–6 min (Haraguchi et al., 2001). Interestingly, emerin recruitment to the ‘core’ region is also necessary for its reassembly into the nuclear envelope (Haraguchi et al., 2001). Whereas emerin itself is not essential for nuclear assembly, LEM-domain proteins are collectively essential since co-downregulation of both Ce-emerin and Ce-lem-2 blocks nuclear envelope assembly in C. elegans (Margalit et al., 2005). However these mitotic roles may not be directly relevant to EDMD disease, since loss of emerin alone does not disrupt mitosis.
Conclusions
There is growing evidence that emerin has both architectural and gene-regulatory roles in the nucleus. However, no single emerin-binding partner can fully explain the EDMD disease mechanism. We propose that each of emerin’s multiple roles might contribute to pathology, and the actual mechanism might differ between tissues. Furthermore, mechanical signaling pathways that integrate nuclear architecture and gene expression, such as the defective mechano-sensitive gene expression seen in Lmna-null cells (Lammerding et al., 2004), might be key to understanding the EDMD-disease mechanism.
Acknowledgements
This work was supported by grants from the American Heart Association (to K.L.W.) and National Institute of Health F32 GM067397 (to J.M.H.).
References
- Bengtsson L, Wilson KL. Multiple and surprising new functions for emerin, a nuclear membrane protein. Curr Opin Cell Biol. 2004;16:73–79. doi: 10.1016/j.ceb.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Bonne G, Yaou RB, Beroud C, Boriani G, Brown S, de Visser M, Duboc D, Ellis J, Hausmanowa-Petrusewicz I, Lattanzi G, Merlini L, Morris G, Muntoni F, Opolski G, Pinto YM, Sangiuolo F, Toniolo D, Trembath R, van Berlo JH, van der Kooi AJ, Wehnert M. 108th ENMC International Workshop, 3rd Workshop of the MYO-CLUSTER project: EUROMEN, 7th International Emery-Dreifuss Muscular Dystrophy (EDMD) Workshop, 13–15 September 2002, Naarden, The Netherlands. Neuromuscul Disord. 2003;13:508–515. doi: 10.1016/s0960-8966(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Burke B, Stewart CL. Life at the edge: the nuclear envelope and human disease. Nat Rev Mol Cell Biol. 2002;3:575–585. doi: 10.1038/nrm879. [DOI] [PubMed] [Google Scholar]
- Clements L, Manilal S, Love DR, Morris GE. Direct interaction between emerin and lamin A. Biochem Biophys Res Commun. 2000;267:709–714. doi: 10.1006/bbrc.1999.2023. [DOI] [PubMed] [Google Scholar]
- Cohen M, Lee KK, Wilson KL, Gruenbaum Y. Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem Sci. 2001;26:41–47. doi: 10.1016/s0968-0004(00)01727-8. [DOI] [PubMed] [Google Scholar]
- de la Luna S, Allen KE, Mason SL, La Thangue NB. Integration of a growth-suppressing BTB/POZ domain protein with the DP component of the E2F transcription factor. EMBO J. 1999;18:212–228. doi: 10.1093/emboj/18.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Vlcek S, Foisner R. Review: lamina-associated polypeptide 2 isoforms and related proteins in cell cycle-dependent nuclear structure dynamics. J Struct Biol. 2000;129:335–345. doi: 10.1006/jsbi.2000.4212. [DOI] [PubMed] [Google Scholar]
- Emery AE. Emery-Dreifuss muscular dystrophy-a 40 year retrospective. Neuromuscul Disord. 2000;10:228–232. doi: 10.1016/s0960-8966(00)00105-x. [DOI] [PubMed] [Google Scholar]
- Fairley E, Kendrick-Jones J, Ellis J. The Emery-Dreifuss muscular dystrophy phenotype arises from aberrant targeting and binding of emerin at the inner nuclear membrane. J Cell Sci. 1999;112:2571–2582. doi: 10.1242/jcs.112.15.2571. [DOI] [PubMed] [Google Scholar]
- Fidzianska A, Hausmanowa-Petrusewicz I. Architectural abnormalities in muscle nuclei. Ultrastructural differences between X-linked and autosomal dominant forms of EDMD. J Neurol Sci. 2003;210:47–51. doi: 10.1016/s0022-510x(03)00012-1. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Goldman AE, Shumaker DK. Nuclear lamins: building blocks of nuclear structure and function. Novartis Found Symp. 2005;264:3–16. discussion 16–21, 227-30. [PubMed] [Google Scholar]
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Holaska JM, Yamane M, Wilson KL, Hiraoka Y. Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery-Dreifuss muscular dystrophy. Eur J Biochem. 2004;271:1035–1045. doi: 10.1111/j.1432-1033.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Koujin T, Segura-Totten M, Lee KK, Matsuoka Y, Yoneda Y, Wilson KL, Hiraoka Y. BAF is required for emerin assembly into the reforming nuclear envelope. J Cell Sci. 2001;114:4575–4585. doi: 10.1242/jcs.114.24.4575. [DOI] [PubMed] [Google Scholar]
- Hartmann AM, Nayler O, Schwaiger FW, Obermeier A, Stamm S. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59(fyn) Mol Biol Cell. 1999;10:3909–3926. doi: 10.1091/mbc.10.11.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska J, Lee K, Kowalski A, Wilson K. Transcriptional repressor germ cell-less (GCL) and barrier-to-autointegration factor (BAF) compete for binding to emerin in vitro. J Biol Chem. 2003;278:6969–6975. doi: 10.1074/jbc.M208811200. [DOI] [PubMed] [Google Scholar]
- Holaska JM, Kowalski AM, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS. 2004;2:1354–1362. doi: 10.1371/journal.pbio.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska JM, Wilson KL, Mansharamani M. The nuclear envelope, lamins and nuclear assembly. Curr Opin Cell Biol. 2002;14:357–364. doi: 10.1016/s0955-0674(02)00329-0. [DOI] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi G, Cenni V, Marmiroli S, Capanni C, Mattioli E, Merlini L, Squarzoni S, Maraldi NM. Association of emerin with nuclear and cytoplasmic actin is regulated in differentiating myoblasts. Biochem Biophys Res Commun. 2003;303:764–770. doi: 10.1016/s0006-291x(03)00415-7. [DOI] [PubMed] [Google Scholar]
- Lee K, Wilson K. All in the family: evidence for four new LEM-domain proteins Lem2 (NET-25), Lem3, Lem4 and Lem5 in the human genome. Oxford, UK: BIOS Scientific Publishers LTD; 2004. [PubMed] [Google Scholar]
- Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol Biol Cell. 2000;11:3089–3099. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Haraguchi T, Lee RS, Koujin T, Hiraoka Y, Wilson KL. Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J Cell Sci. 2001;114:4567–4573. doi: 10.1242/jcs.114.24.4567. [DOI] [PubMed] [Google Scholar]
- Libotte T, Zaim H, Abraham S, Padmakumar VC, Schneider M, Lu W, Munck M, Hutchison C, Wehnert M, Fahrenkrog B, Sauder U, Aebi U, Noegel AA, Karakesisoglou I. Lamin A/C Dependent Localization of Nesprin-2, a Giant Scaffolder at the Nuclear Envelope. Mol Biol Cell. 2005;16(7):3411–3424. doi: 10.1091/mbc.E04-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum Mol Genet. 2005;14:437–445. doi: 10.1093/hmg/ddi040. [DOI] [PubMed] [Google Scholar]
- Liu J, Lee KK, Segura-Totten M, Neufeld E, Wilson KL, Gruenbaum Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100:4598–4603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manilal S, Nguyen TM, Morris GE. Colocalization of emerin and lamins in interphase nuclei and changes during mitosis. Biochem. Biophys. Res. Commun. 1998;249:643–647. doi: 10.1006/bbrc.1998.9209. [DOI] [PubMed] [Google Scholar]
- Manilal S, Nguyen TM, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum. Mol. Genet. 1996;5:801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- Mansharamani M, Wilson KL. Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem. 2005;280:13863–13870. doi: 10.1074/jbc.M413020200. [DOI] [PubMed] [Google Scholar]
- Maraldi NM, Lattanzi G, Marmiroli S, Squarzoni S, Manzoli FA. New roles for lamins, nuclear envelope proteins and actin in the nucleus. Adv Enzyme Regul. 2004;44:155–172. doi: 10.1016/j.advenzreg.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Margalit A, Segura-Totten M, Gruenbaum Y, Wilson KL. Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc Natl Acad Sci U S A. 2005;102:3290–3295. doi: 10.1073/pnas.0408364102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E, Venables R, Mauricio Alvarez R, Quinlan R, Dorobek M, Hausmanowa-Petrucewicz I, Hutchison C. Increased solubility of lamins and redistribution of lamin C in X-linked Emery-Dreifuss muscular dystrophy fibroblasts. J Struct Biol. 2002;140:241–253. doi: 10.1016/s1047-8477(02)00573-7. [DOI] [PubMed] [Google Scholar]
- Mislow JMK, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, McNally EM. Nesprin-1α self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002a;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- Mislow JMK, Kim MS, Davis DB, McNally EM. Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C. J Cell Sci. 2002b;115:61–70. doi: 10.1242/jcs.115.1.61. [DOI] [PubMed] [Google Scholar]
- Muchir A, van Engelen BG, Lammens M, Mislow JM, McNally E, Schwartz K, Bonne G. Nuclear envelope alterations in fibroblasts from LGMD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp Cell Res. 2003;291:352–362. doi: 10.1016/j.yexcr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Nagano A, Koga R, Ogawa M, Kurano Y, Kawada J, Okada R, Hayashi YK, Tsukahara T, Arahata K. Emerin deficiency at the nuclear membrane in patients with Emery- Dreifuss muscular dystrophy. Nat. Genet. 1996;12:254–259. doi: 10.1038/ng0396-254. [DOI] [PubMed] [Google Scholar]
- Nili E, Cojocaru GS, Kalma Y, Ginsberg D, Copeland NG, Gilbert DJ, Jenkins NA, Berger R, Shaklai S, Amariglio N, Brok-Simoni F, Simon AJ, Rechavi G. Nuclear membrane protein LAP2beta mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less) J Cell Sci. 2001;114:3297–3307. doi: 10.1242/jcs.114.18.3297. [DOI] [PubMed] [Google Scholar]
- Osada S, Ohmori SY, Taira M. XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development. 2003;130(9):1783–1794. doi: 10.1242/dev.00401. [DOI] [PubMed] [Google Scholar]
- Ostlund C, Worman HJ. Nuclear envelope proteins and neuromuscular diseases. Muscle Nerve. 2003;27:393–406. doi: 10.1002/mus.10302. [DOI] [PubMed] [Google Scholar]
- Pederson T, Aebi U. Actin in the nucleus: what form and what for? J Struct Biol. 2002;140:3–9. doi: 10.1016/s1047-8477(02)00528-2. [DOI] [PubMed] [Google Scholar]
- Raju GP, Dimova N, Klein PS, Huang HC. SANE, a novel LEM domain protein, regulates BMP signaling through interaction with Smad1. J Biol Chem. 2003;278:428–437. doi: 10.1074/jbc.M210505200. [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- Segura-Totten M, Kowalski AM, Craigie R, Wilson KL. Barrier-to-autointegration factor: major roles in chromatin decondensation and nuclear assembly. J Cell Biol. 2002;158:475–485. doi: 10.1083/jcb.200202019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Totten M, Wilson K. BAF: roles in chromatin, nuclear structure and retrovirus integration. Trends Cell Biol. 2004;14:261–266. doi: 10.1016/j.tcb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shimi T, Koujin T, Segura-Totten M, Wilson KL, Haraguchi T, Hiraoka Y. Dynamic interaction between BAF and emerin revealed by FRAP, FLIP, and FRET analyses in living HeLa cells. J Struct Biol. 2004;147:31–41. doi: 10.1016/j.jsb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Somech R, Shaklai S, Amariglio N, Rechavi G, Simon AJ. Nuclear envelopathies--raising the nuclear veil. Pediatr Res. 2005;57:8R–15R. doi: 10.1203/01.PDR.0000159566.54287.6C. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Escalente-Alcalde D, Bhatt H, Anver M, Naryan B, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Tsujino S, Arahata K. CDNA microarray analysis of gene expression in fibroblasts of patients with X-linked Emery-Dreifuss muscular dystrophy. Muscle Nerve. 2002;25:898–901. doi: 10.1002/mus.10085. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu S, Rivolta C, Li LY, Peng G, Swain PK, Sung C, Swaroop A, Berson EL, Dryja TP, Chen S. Barrier to autointegration factor interacts with the cone-rod homeobox and represses its transactivation function. J Biol Chem. 2002;277:43288–43300. doi: 10.1074/jbc.M207952200. [DOI] [PubMed] [Google Scholar]
- Warren DT, Zhang Q, Weissberg PL, Shanahan CM. Nesprins: intracellular scaffolds that maintain cell architecture and coordinate cell function? Expert Rev Mol Med. 2005;7:1–15. doi: 10.1017/S1462399405009294. [DOI] [PubMed] [Google Scholar]
- Wilkinson FL, Holaska JM, Sharma A, Malinal SB, Holt I, Stamm S, K.L. W, Morris GE. Emerin interacts in vitro with the splicing-associated factor, YT521-B. Eur J Biochem. 2003;270:2459–2466. doi: 10.1046/j.1432-1033.2003.03617.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114(Pt 24):4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]