Abstract
Brain derived neurotrophic factor and neurotrophin-4 are required for normal taste bud development. Although these neurotrophins normally function via the tyrosine kinase receptor, trkB, they also bind to the pan-neurotrophin receptor, p75. The goal of the present study was to determine whether the p75 receptor is required for the development or maintenance of a full complement of adult taste buds. Mice with p75 null mutations lose 34% of their circumvallate taste buds, 36% of their fungiform papillae, and 26% of their fungiform taste buds by adulthood. The reduction of taste buds in the adult circumvallate papilla was similar to that observed previously at postnatal day 7 ((Fan et al., 2004). Taken together, these findings indicate that the p75 receptor is critical for the development of a full complement of taste buds, but is not required for maintenance of circumvallate taste buds in adulthood. Immunolabeling for p75 was not observed in taste buds, indicating that p75 signaling influences taste bud number indirectly. However, geniculate ganglion neurons, which provides innervation to fungiform taste buds, express the p75 receptor. Mice with p75-null mutations also have fewer neurons in the geniculate ganglion. Together, these results suggest that survival of geniculate neurons is essential for the development of a full complement of taste buds.
Keywords: taste buds, p75 receptor, neurotrophin, geniculate ganglion
INTRODUCTION
Normal taste system development is dependent upon neurotrophins, a group of structurally related growth factors that influence numerous aspects of sensory neuron development. In sensory ganglia, neurotrophins regulate early cell cycle events and survival of neuronal precursors (for a review see (Huang and Reichardt, 2001). Neurotrophins produced along axonal trajectories regulate neurite outgrowth and branching. They also influence survival of neurons before they reach their targets. Target derived neurotrophins, such as those derived from taste buds, can regulate neuronal differentiation and survival, target invasion, and synapse formation. Lastly, neurotrophins can maintain sensory innervation and/or regulate neural function in adults.
Gustatory neurons in the geniculate and petrosal ganglia require neurotrophins for survival during development. Specifically, mice lacking brain-derived neurotrophic factor (BDNF) or neurotrophin-4 (NT4) lose half of their geniculate and petrosal ganglion neurons by birth (Jones et al., 1994; Conover et al., 1995; Liu et al., 1995; Liebl et al., 1997). Sensory innervation of the fungiform and circumvallate papillae is also reduced in mice lacking BDNF (BDNF−/−). Consequently, fungiform and circumvallate taste buds are lost in BDNF null mice (Nosrat et al., 1997; Zhang et al., 1997; Cooper and Oakley, 1998; Mistretta et al., 1999). Fungiform papillae are lost in NT4−/− mice, but circumvallate papilla are normal (Liebl et al., 1999). In addition, mice that overexpress BDNF or NT4 in the lingual epithelium have twice the number of geniculate neurons normally observed in adults (Krimm et al., 2001). These findings demonstrate that BDNF and NT4 are required for survival of gustatory neurons and taste buds during development. Due to the severity of the developmental defects associated with the removal of BDNF and NT4, it is unclear whether these neurotrophins are also important for maintaining the gustatory system in adulthood.
Neurotrophins mediate their biological effects via the receptor tyrosine kinases, trkA, trkB, and trkC, or the p75 receptor, a member of the tumor necrosis super-family. The role of p75 is enigmatic primarily because this receptor interacts with such a diverse group of molecules, resulting in a large array of downstream events (for review see (Huang and Reichardt, 2001; Bronfman and Fainzilber, 2004; Yamashita et al., 2005a). For example, the p75 receptor has been shown to enhance neuron survival in some instances (Stucky and Koltzenburg, 1997; DeFreitas et al., 2001; Song and Posse de Chaves, 2003) and to mediate cell death in others (Agerman et al., 2000; Botchkarev et al., 2000; Lee et al., 2001; Troy et al., 2002). Axon growth (Yamashita et al., 1999; Bentley and Lee, 2000; Gallo and Letourneau, 2004; Gehler et al., 2004) and axonal regeneration (Boyd and Gordon, 2001; Song et al., 2004; Song et al., 2005; Yamashita et al., 2005b) are also differentially regulated by this receptor.
The p75 receptor may also be important for maintaining sensory receptor cells and their innervation during both late postnatal development and in adulthood. Mice lacking p75 (p75−/−) have sensory neuron and receptor deficits and develop periodic lesions on their footpads (Lee et al., 1992). A specific receptor complex, comprised of specialized p75 receptor expressing cells of the epidermis known as Merkel cells (English et al., 1994; Szeder et al., 2003; Sieber-Blum et al., 2004) and slowly adapting type 1 (SA1) mechanosensory neurons, requires the p75 receptor for its maintenance during late postnatal development (Fundin et al., 1997; Stucky and Koltzenburg, 1997; Rice et al., 1998; Kinkelin et al., 1999). In p75−/− animals, Merkel cells are lost between 2 weeks and 2 months of age (Fundin et al., 1997; Kinkelin et al., 1999; Cronk et al., 2002). Merkel cells express the p75 receptor (English et al., 1994; Szeder et al., 2003; Sieber-Blum et al., 2004) and in some instances Merkel cell loss appears to be a direct effect of p75 signaling in Merkel cells and is not due to loss of innervation (Kinkelin et al., 1999). Thus, the p75 receptor is important for late postnatal development and/or adult maintenance of some sensory receptor cells and their associated innervation.
It is likely that the p75 receptor may play a similar role in the gustatory system. The p75 receptor is expressed in taste bud cells (Fan et al., 2004; Yee et al., 2005), and a small proportion of taste buds in the circumvallate papillae are lost by postnatal day 7 (P7) in p75−/− mice (Fan et al., 2004). However, most peripheral somatosensory deficits in p75−/− mice (e.g., Merkel cell loss and deficits in whisker pad sensory innervation) do not occur until after PN7. Furthermore, taste buds continue to be added in the circumvallate papillae after PN7 {Hosley, 1987 #59} and the p75 receptor could required for this postnatal acquisition of taste buds. To determine if the p75 receptor has a role maintaining taste buds during postnatal development and adulthood, we quantified the loss of circumvallate in adult p75-null mutants to compare with losses already reported at PN7 (Fan et al., 2004). If the p75 receptor is important for the postnatal maintenance of taste buds after PN7, then the absence of p75 should have a much greater impact on taste bud number by adulthood then by PN7. This article also extends these earlier findings by determining whether fungiform papillae and taste buds also require p75 for either development or adult maintenance. Whether the reduction in taste buds is also accompanied by a reduction of neurons in the gustatory ganglion that provides innervation to fungiform taste buds, was also determined. It was hypothesized that p75−/−mice would have fewer fungiform taste buds and geniculate ganglion neurons than wild type mice.
METHODS
Animals
The p75−/− mice employed here have a homozygous deletion in the third exon of the p75 gene (Lee et al., 1992), which eliminates the full-length p75 receptor but not the short isoform (von Schack et al., 2001). The short isoform does not bind neurotrophins and, therefore, cannot mediate the functions of these factors. However, this isoform may have other, neuotrophin-independent roles. This is the same deletion examined in all aforementioned taste and somatosensory studies (Fundin et al., 1997; Stucky and Koltzenburg, 1997; Kinkelin et al., 1999; Fan et al., 2004). The p75 knockout mice were generated on a mixed 129 X BALB/C genetic background (Lee et al., 1992) and were obtained from DNX Transgenic Sciences (Princeton, NJ). For another study, these p75−/− mice were bred with mice having a mixed C3H X C57BL6 background. Results from the p75−/− mice were compared with age matched wild-type mice of same mixed background. Animals were used in accordance with the guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
Wild type mice (n=3) were deeply anesthetized with 2.5% avertin diluted in 0.9% saline (0.02 ml/gm body weight) and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. Tongues were embedded in 10% gelatin and post-fixed overnight in 4% paraformaldehyde. Gelatin-embedded tissue was infiltrated with 30% sucrose in phosphate buffered saline (PBS), frozen, and sectioned at 40μm on a sliding microtome. Geniculate ganglia were placed in 4% paraformaldehyde overnight, followed by 30% sucrose overnight. They were then frozen in OCT and sectioned on the cryostat at 12 μm. Tissue sections were washed in PBS, blocked for 1 hr at room temperature in 5% normal goat serum, 2% bovine serum albumin, and 0.25% Triton X-100. Sections were incubated overnight in a rabbit polyclonal antisera (No. 9992; 1:1000) directed against the p75 cytoplasmic domain (Casaccia-Bonnefil et al., 1996), which was a generous gift from M. Chao (Cornell University Medical College, NY). Sections were then washed, incubated with goat anti-rabbit biotinylated antibody (1:500; Vector Laboratories, Burlingame, CA) for 1 hr, re-washed, and treated with a solution containing 2.5% H202 and 5% methanol to block endogenous peroxidases. For antibody detection, tissues were incubated in an avidin-biotin-complex mix (ABC, Vectastain Elite; Vector Laboratories) for 1 hr, washed, incubated in nickel-enhanced 0.04% diaminobenzidene solution, mounted onto slides, and counter stained with methyl-green (tongues) or cresyl violet (geniculate ganglia).
In situ mRNA hybridization
Sense and antisense RNA riboprobes were generated to cDNA sequences encoding the p75 receptor (Kitzman et al., 1998). Geniculate ganglia were frozen on dry ice (4 ganglia from 2 mice), cut into 15 μm sections, and thaw mounted onto Superfrost slides. Sections were brought to room temperature, immersion-fixed for 15 min in 4% paraformaldehyde in PBS, and successively washed in PBS and in PBS containing 0.2% glycine and 0.25% acetic anhydride in 0.1 M TEA, pH 8.0. Sections were dehydrated, air-dried, and incubated with probe hybridization solution (Amresco, Solon, OH) containing 1 × 106 cpm/50 μl. A glass coverslip was placed over the probe and secured to the slide by applying mounting media on the edges of the coverslip. Slides were incubated overnight at 60°C, dipped in NTB2 photographic emulsion, exposed 1–2 weeks, developed, and counterstained with hematoxylin and eosin. Sense transcript controls processed in parallel showed no specific hybridization.
Fungiform papillae number
Paraformaldehyde-fixed tongues from wild type (n=4) and p75−/− mice (n=5) were washed in phosphate buffer. The muscle underneath the epithelial surface was then removed, and the surface was stained with a concentrated solution of methylene blue. The epithelial sheet was mounted on a glass slide using glycerol. A drawing tube attached to an Olympus microscope (Olympus Corp., Lake Success, NY) was used to trace maps of the tongue surface to localize fungiform papilla, the intermolar eminence, and borders of the tongue.
Taste bud number and size
Paraformaldehyde-fixed tissue blocks containing the posterior tongue from wild type (n=3) and p75−/− mice (n=3) were dissected expose the circumvallate papilla and some surrounding tissue. The anterior tongue was hemisected at the midline and examined for fungiform taste bud number and size. Tissues were paraffin embedded, serially sectioned at 8 μm, and stained with hematoxylin and eosin. For circumvallate and nasoincisive papillae, sections were examined for taste bud-containing pores. Each fungiform papillae was examined to determine whether or not it had a taste bud and the number of taste buds per papilla was recorded. To determine the volume occupied by each taste bud, the area of each fungiform taste bud was measured (NIH image), multiplied by the section thickness, and summed across sections containing the taste bud.
Geniculate ganglion neuron number and size
Ganglia were serially sectioned at 5 μm and Nissl stained. Every 5th section was examined at 400X for neuronal profiles with a nucleus. To estimate the total number of neuron profiles with a nucleus in all sections, the number of nuclear profiles that were counted was multiplied by five. To estimate the total number of neurons the number of neuron profiles was multiplied by a correction factors to compensate for the presence of the nucleus in multiple sections. The total number of nuclear profiles was multiplied by the Abercrombie correction factor (N = n × T/T + D), where N is the estimated total number of cells, n is the number of nuclear profiles, T is the section thickness, and D is the average diameter of nuclei. This correction factor was calculated separately for each ganglion based on the average nuclear diameter of 50 neurons and the average thickness of six sections. Diameters were calculated from area measurements of each neuron or nucleus (NIH image). The mean diameter of 100 neurons per ganglion was compared across genotypes.
Statistical analysis
Comparisons of group means were performed using Student’s t-test. P < 0.05 was accepted as significant. All data are expressed as mean ± standard error of the mean.
RESULTS
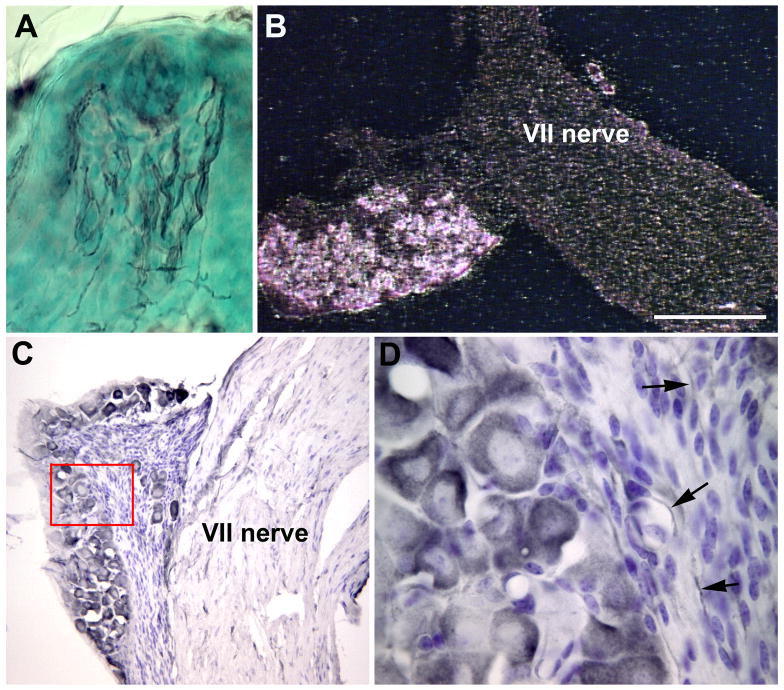
It has been reported that the p75 receptor is present not only in peripheral sensory neurons, but also in somatosensory receptor cells (i.e. Merkel cells) and taste cells (Fan et al., 2004; Yee et al., 2005). To verify these findings in the taste system, tongue sections were immunohistochemically labeled for the p75 receptor. In contrast to previous reports, we did not observe any specific labeling in taste bud cells (Figure 1A). However, neural fibers throughout the fungiform taste buds and in non-taste bud containing regions of the fungiform papillae were labeled. The cell bodies of fibers that provide gustatory innervation to fungiform taste buds are located in the geniculate ganglion. In situ hybridization and immunohistochemistry revealed that most geniculate neurons expressed the p75 receptor (Figure 1B, C). The intensity of p75-like immunoreactivity varied between cells (Figure 1 C). Immunolabeling for p75 was also observed in nerve fibers within the geniculate ganglion (Figure 1D, arrows), but there did not appear to be any p75-like immunoreactivity in satellite cells. Thus, the p75 receptor is localized to gustatory neurons, but not taste receptor cells.
Figure 1.
Gustatory neurons express the p75 receptor. A fungiform papilla (A) labeled with an antibody to p75 demonstrates labeling of nerve fibers in the papilla core as well as in the taste bud (black arrows). A darkfield image of the geniculate ganglion following p75 in situ hybridization (B) illustrates that most neurons in the geniculate ganglion (between arrows) express p75 mRNA. A geniculate ganglion labeled with an antibody to p75 (C) demonstrates that all the ganglion neurons contain this protein although some are more intensely labeled than others. The area outlined by the red box in C is shown at higher magnification (D). p75 labeling can be seen in the cytoplasm of all neurons, but not in the satellite cells. Some p75-labeled fibers can also be identified in the nerve bundles (arrows). Scale bar in B = 250 µm.
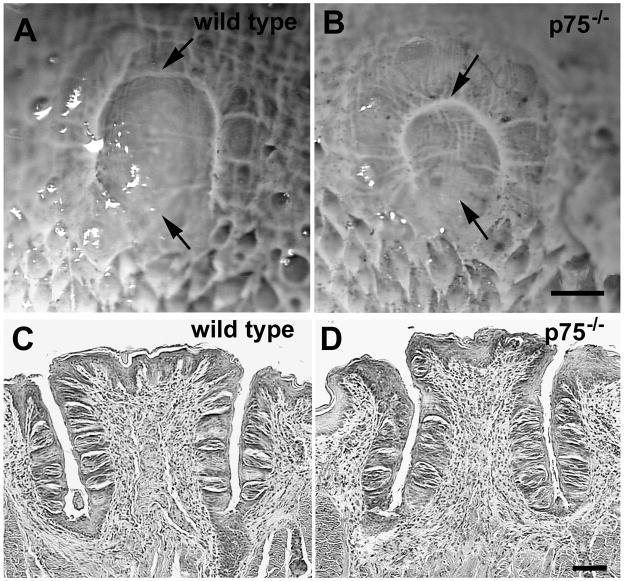
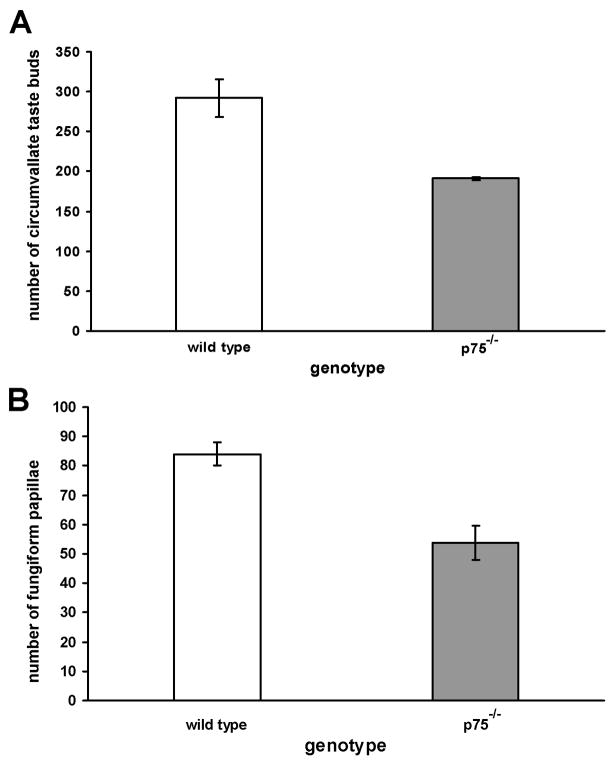
To determine if the loss of circumvallate taste buds in adult p75−/− mice was greater than that previously observed at P7, the number of taste buds in the circumvallate papillae of adult p75−/− and wild type mice was quantified. The circumvallate papilla was noticeably shorter along its rostral-caudal extent in p75−/− mice than in wild type mice (Figure 2A compared with B). As a result, the circumvallate papilla occupied fewer sections in p75−/− mice compared with wild type mice. The depth of circumvallate trenches appeared to be similar in both genotypes (Figure 2D compared with C), and no dramatic changes in trench wall taste bud density were observed in knockout animals. There were, however, 34% fewer circumvallate taste buds in p75−/− mice compared with wild type mice (Figure 3A, p < 0.01).
Figure 2.
The circumvallate papilla is smaller in p75−/− mice than in wild type mice. When viewed from the dorsal surface, the rostral to caudal length of the circumvallate papillae (between arrows) is shorter in a p75−/− mouse (B) than in a wild type mouse (A). H&E-stained cross sections through the center of the circumvallate papilla illustrate the trench depth of the papilla is similar in a wild type (D) and p75−/− (C) mouse. In addition, the trenches are lined with taste buds in both genotypes. Scale bar in D = 250 µm and applies to C and D. Scale bar in F = 50 µm and applies to E and F.
Figure 3.
Circumvallate taste buds and fungiform papillae are reduced in p75−/− mice. The number of circumvallate taste buds (A) was determined by counting taste pores in serially sectioned circumvallate papillae stained with H&E. The total number of fungiform papillae (B) was obtained from tongue maps of wild type (n = 4) and p75−/−mice (n = 5) following staining of the surface with methylene blue. Knockout animals had fewer fungiform papillae (p < 0.005) and fewer circumvallate taste buds (p < 0.01) than their wild type counterparts did.
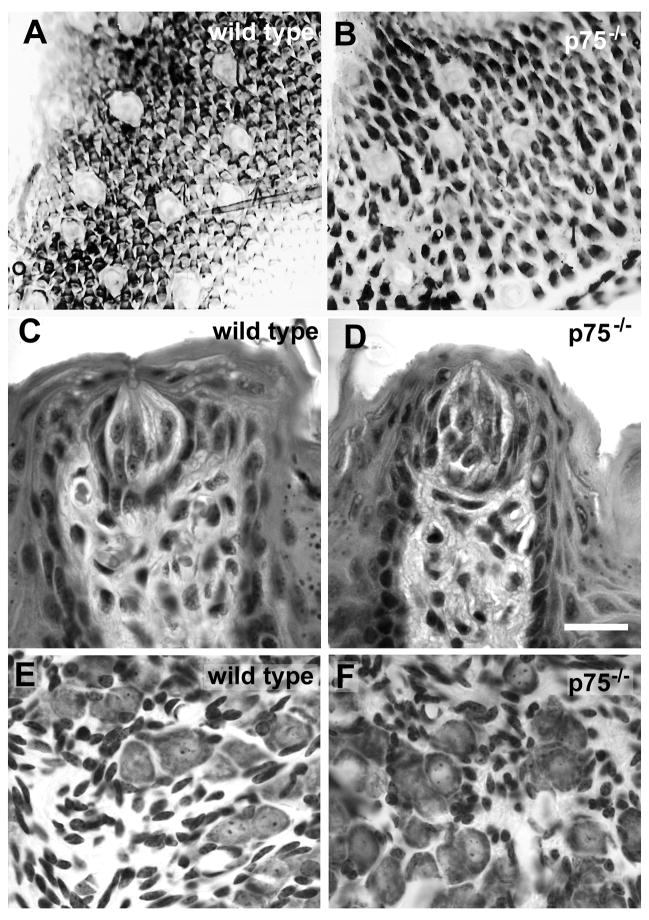
Next, we examined the effect of p75 removal on the number of fungiform papillae and taste buds. Fungiform papillae were quantified by labeling the surface epithelium of the tongue with methylene blue as described in Methods (Figure 4A, B). Knockout animals had 36% fewer fungiform papillae than their wild type counterparts (Figure 3B, p < 0.005). Thus, the p75 receptor is required for development of a full complement of fungiform papillae. In a separate set of animals, the number of taste buds/fungiform papillae was examined in one-half of the serially sectioned tongue. Taste bud number was decreased by 26% in p75−/− mice (wild type, 46 ± 1.4 vs. p75−/−, 34 ± 3.7; p = 0.04). All fungiform papillae examined contained at least one taste bud with two exceptions. One wild type and one p75−/− animal had several papillae with two taste buds. There was no difference in the number of taste buds/fungiform papilla in wild type (1.01 ± .01) and p75−/− mice (1.04 ± 0.04; p = 0.5). Furthermore, taste volumes of the remaining taste buds did not differ between wild type (Figure 4C; 15,190 ± 1253 μm3) and p75−/− mice (Figure 4D, 17,913 ± 4280 μm3; p = 0.57).
Figure 4.
There are fewer fungiform papillae in p75−/− mice than wild type mice. The surface of the tongue tip from a wild type (A) and a p75−/− mouse (B) stained with methylene blue. The fungiform papillae are clear and easily quantified. The size of fungiform taste buds is unaffected by deletion of p75 (D compared with C). The remaining geniculate ganglion neurons are the same size in p75−/− mice as in wild type mice (F compared with E).
The loss of fungiform papillae and taste buds could be attributable to a loss of geniculate ganglion neurons. A comparison of the number of geniculate neurons in both genotypes revealed that mice lacking p75 had 25% fewer geniculate ganglion neurons (554 ± 31) than wild type mice (742 ± 47, p < 0.03). The diameter of the remaining geniculate neurons was normal (Figure 4 E, F; wild type, 12.1 ± 1.3 µm vs. p75−/−, 13.4 ± 1.5 μm; p = 0.24).
DISCUSSION
Gustatory development relies upon the neurotrophins, BDNF and NT4, which function via two receptors, trkB and the pan-neurotrophin receptor, p75. It has already been established that the trkB receptor is required for normal gustatory development (Fritzsch et al., 1997). The purpose of this study was to determine whether the p75 receptor is also required for maintaining a full complement of taste buds into adulthood. Adult p75−/− mice have 34% fewer taste buds in the circumvallate papillae. A similar decrease was observed on P7 (Fan et al., 2004), suggesting that taste bud loss neither increases nor is compensated for during postnatal development. Fungiform papillae and taste buds were also comparably reduced in adult p75−/− mice. Remaining taste buds were normal in size. Together, these results suggest that the p75 receptor is required for the normal development or maintenance of a full complement of taste buds.
BDNF is produced in developing gustatory epithelia and taste buds, while NT3 is produced by epithelia surrounding the taste buds(Nosrat and Olson, 1995; Nosrat et al., 1996; Nosrat, 1998; Nosrat and Olson, 1998; Nosrat et al., 2001). It is possible that neurotrophins produced in or around taste buds bind directly to p75 receptors and influence taste buds via autocrine or paracrine mechanisms. This type of nerve-independent mechanism functions to maintain adult Merkel cells (English et al., 1994; Kinkelin et al., 1999; Sieber-Blum et al., 2004). However, it is unlikely that neurotrophins act directly on taste buds via p75, since unlike previous reports (Fan et al., 2004; Yee et al., 2005), no p75-like immunoreactivity was observed in taste cells. Rather, it is possible that taste buds are lost in p75−/− mice because p75 expressing gustatory neurons are lost. Consistent with this possibility we did observe robust p75-LIR was present in gustatory and somatosensory neuronal fibers and in geniculate neuron cell bodies. To evaluate this possibility, we counted the number of geniculate neurons in adult p75−/− and wild type mice. We found 25% less geniculate neurons in p75−/− mice compared with wild type mice. This reduction was similar to that reported previously (Fan et al., 2004). Loss of gustatory innervation during development can reduce the numbers of taste buds and fungiform papillae (Hosley et al., 1987; Nagato et al., 1995; Sollars and Bernstein, 2000; Sollars et al., 2002; Sollars, 2005). Thus, one explanation for the present findings is that neurotrophins function to maintain gustatory neurons via the p75 receptor. Innervation from these neurons is important for the development and/or maintenance of a full complement of taste buds.
It is unclear whether a specific subpopulation of geniculate neurons is lost in the p75−/− mice. The geniculate ganglion provides gustatory innervation to taste buds within fungiform papillae on the front of the tongue and to the taste buds within the palate. In addition, it contains somatosensory neurons that innervate the outer ear. It is possible that all subpopulations are equally impacted by deletion of the p75 receptor. All functional classes of dorsal root ganglion (DRG) neurons are lost to the same degree in mice lacking a functional p75 receptor (Stucky and Koltzenburg, 1997). The remaining geniculate ganglion neurons did not exhibit any alterations in diameter; this indicates that the missing neurons are evenly distributed across different size subpopulations. Therefore, it is likely that the p75 receptor plays a general role in supporting sensory neuron survival.
In addition to supporting gustatory neuron survival, the p75 receptor may also regulate proper targeting of gustatory neurons to fungiform papillae and taste buds. Within the lingual epithelium, BDNF is specifically expressed in fungiform papillae. This expression pattern is critical for successful innervation of taste epithelia by gustatory neurons (Ringstedt et al., 1999; Krimm et al., 2001), suggesting that BDNF plays a chemotactic role during the final stages of axon targeting. The p75 receptor is also important for neurotrophin regulation of growth cone dynamics in vitro (Gallo and Letourneau, 2004; Gehler et al., 2004). It is possible that BDNF-mediated gustatory axon targeting is disrupted in p75−/− mice. If so, the usual complement of gustatory neurons may not innervate fungiform papillae, resulting in reduced numbers of taste buds.
Taste buds continue to be added to the circumvallate papillae throughout postnatal development (Hosley et al., 1987). At one week of age, wild type mice have approximately 105 taste buds in the circumvallate papillae (Fan et al., 2004). By 4 months, this number is more than doubled (292 taste buds). Taste buds also continue to be added to the circumvallate of p75−/− mice during postnatal development. More than twice as many taste buds were present in adults (191) than in P7 mice (78). Because taste buds are added during postnatal development in wild type and p75−/− mice, the proportional loss of taste buds in adult p75−/− mice (34%) was roughly the same as that observed during development (26%). If p75 receptors play a major role maintaining taste buds during postnatal development, taste bud loss in adults should be substantially greater than at P7. However, this was not the case, indicating that the role of p75 in the taste system is primarily developmental. Loss of taste buds in the circumvallate papilla of p75−/− mice has been observed at birth (Fan et al., 2004). However, the time during embryonic development at which such losses occur is unclear.
Circumvallate and fungiform taste buds were both lost in adult p75−/− mice, indicating that the p75 receptor is important for the development of more than one subpopulation of taste buds. We did not observe any fungiform papillae lacking taste buds. Therefore, all the taste buds that were lost were associated with the loss of the corresponding fungiform papilla. This finding is consistent with a developmental role for p75. Removal of gustatory innervation during development results in the loss of both fungiform papillae and taste buds, while removal of innervation during adulthood causes taste bud loss but does not affect the fungiform papilla (Oakley et al., 1990; Nagato et al., 1995; Sollars, 2005). Quantification of fungiform taste buds over the course of development will be necessary to provide unequivocal evidence for a developmental role for p75 for fungiform papillae and taste buds.
In conclusion, p75 appears to function in the taste system by enhancing neuronal survival during development. Unlike the p75-expressing Merkel cells, we did not observe p75-like immunoreactivity in adult taste buds. Also in contrast to Merkel cells, which are lost after P14, we did not observe any loss of circumvallate taste buds in p75 null adults beyond that which has occurred by P7. These findings indicate that the p75 receptor does not have a direct role in postnatal maintenance of taste buds. Instead, the early loss of gustatory neurons in p75−/− mice prevents the circumvallate papillae from developing a full complement of taste buds by adulthood. Removal of p75 has the same effects on fungiform taste buds as it does on circumvallate taste buds. Therefore, p75 plays a general role during the development of multiple taste bud populations, as well as in the survival and maintenance of gustatory neurons. Taken together, these findings indicate that although two different types of epidermal sensory cells, taste cells and Merkel cells, both require p75, the role of p75 in supporting these two cell types is undoubtedly different.
Acknowledgments
Grant sponsor: National Institutes of Health Grant number: DC004763 and DC007176.
References
- Agerman K, Baudet C, Fundin B, Willson C, Ernfors P. Attenuation of a caspase-3 dependent cell death in NT4- and p75-deficient embryonic sensory neurons. Mol Cell Neurosci. 2000;16:258–268. doi: 10.1006/mcne.2000.0875. [DOI] [PubMed] [Google Scholar]
- Bentley CA, Lee KF. p75 is important for axon growth and schwann cell migration during development. J Neurosci. 2000;20:7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Albers KM, Chen LH, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. Faseb J. 2000;14:1931–1942. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J Neurobiol. 2001;49:314–325. doi: 10.1002/neu.10013. [DOI] [PubMed] [Google Scholar]
- Bronfman FC, Fainzilber M. Multi-tasking by the p75 neurotrophin receptor: sortilin things out? EMBO Rep. 2004;5:867–871. doi: 10.1038/sj.embor.7400219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- Cooper D, Oakley B. Functional redundancy and gustatory development in bdnf null mutant mice. Brain Res Dev Brain Res. 1998;105:79–84. [PubMed] [Google Scholar]
- Cronk KM, Wilkinson GA, Grimes R, Wheeler EF, Jhaveri S, Fundin BT, Silos-Santiago I, Tessarollo L, Reichardt LF, Rice FL. Diverse dependencies of developing Merkel innervation on the trkA and both full-length and truncated isoforms of trkC. Development. 2002;129:3739–3750. doi: 10.1242/dev.129.15.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFreitas MF, McQuillen PS, Shatz CJ. A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons. J Neurosci. 2001;21:5121–5129. doi: 10.1523/JNEUROSCI.21-14-05121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English KB, Harper S, Stayner N, Wang ZM, Davies AM. Localization of nerve growth factor (NGF) and low-affinity NGF receptors in touch domes and quantification of NGF mRNA in keratinocytes of adult rats. J Comp Neurol. 1994;344:470–480. doi: 10.1002/cne.903440309. [DOI] [PubMed] [Google Scholar]
- Fan L, Girnius S, Oakley B. Support of trigeminal sensory neurons by nonneuronal p75 neurotrophin receptors. Brain Res Dev Brain Res. 2004;150:23–39. doi: 10.1016/j.devbrainres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Neurosci. 1997;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev Biol. 1997;190:94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58:92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- Gehler S, Gallo G, Veien E, Letourneau PC. p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity. J Neurosci. 2004;24:4363–4372. doi: 10.1523/JNEUROSCI.0404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosley MA, Hughes SE, Morton LL, Oakley B. A sensitive period for the neural induction of taste buds. J Neurosci. 1987;7:2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkelin I, Stucky CL, Koltzenburg M. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. Eur J Neurosci. 1999;11:3963–3969. doi: 10.1046/j.1460-9568.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- Kitzman PH, Perrone TN, LeMaster AM, Davis BM, Albers KM. Level of p75 receptor expression in sensory ganglia is modulated by NGF level in the target tissue. J Neurobiol. 1998;35:258–270. doi: 10.1002/(sici)1097-4695(19980605)35:3<258::aid-neu3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol. 2001;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Mbiene JP, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213:378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Goosens KA, Farinas I, Reichardt LF. Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol. 1999;409:13–24. [PMC free article] [PubMed] [Google Scholar]
- Nagato T, Matsumoto K, Tanioka H, Kodama J, Toh H. Effect of denervation on morphogenesis of the rat fungiform papilla. Acta Anat (Basel) 1995;153:301–309. doi: 10.1159/000147739. [DOI] [PubMed] [Google Scholar]
- Nosrat CA. Neurotrophic factors in the tongue: expression patterns, biological activity, relation to innervation and studies of neurotrophin knockout mice. Ann N Y Acad Sci. 1998;855:28–49. doi: 10.1111/j.1749-6632.1998.tb10544.x. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J Comp Neurol. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, MacCallum DK, Mistretta CM. Distinctive spatiotemporal expression patterns for neurotrophins develop in gustatory papillae and lingual tissues in embryonic tongue organ cultures. Cell Tissue Res. 2001;303:35–45. doi: 10.1007/s004410000271. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J Comp Neurol. 1995;360:698–704. doi: 10.1002/cne.903600413. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Changes in neurotrophin-3 messenger RNA expression patterns in the prenatal rat tongue suggest guidance of developing somatosensory nerves to their final targets. Cell Tissue Res. 1998;292:619–623. doi: 10.1007/s004410051092. [DOI] [PubMed] [Google Scholar]
- Oakley B, Wu LH, Lawton A, deSibour C. Neural control of ectopic filiform spines in adult tongue. Neuroscience. 1990;36:831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev Biol. 1998;198:57–81. [PubMed] [Google Scholar]
- Ringstedt T, Ibanez CF, Nosrat CA. Role of brain-derived neurotrophic factor in target invasion in the gustatory system. J Neurosci. 1999;19:3507–3518. doi: 10.1523/JNEUROSCI.19-09-03507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber-Blum M, Szeder V, Grim M. The role of NT-3 signaling in Merkel cell development. Prog Brain Res. 2004;146:63–72. doi: 10.1016/S0079-6123(03)46004-4. [DOI] [PubMed] [Google Scholar]
- Sollars SI. Chorda tympani nerve transection at different developmental ages produces differential effects on taste bud volume and papillae morphology in the rat. J Neurobiol. 2005;64:310–320. doi: 10.1002/neu.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars SI, Bernstein IL. Neonatal chorda tympani transection permanently disrupts fungiform taste bud and papilla structure in the rat. Physiol Behav. 2000;69:439–444. doi: 10.1016/s0031-9384(99)00259-0. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Smith PC, Hill DL. Time course of morphological alterations of fungiform papillae and taste buds following chorda tympani transection in neonatal rats. J Neurobiol. 2002;51:223–236. doi: 10.1002/neu.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Posse de Chaves EI. Inhibition of rat sympathetic neuron apoptosis by ceramide. Role of p75NTR in ceramide generation. Neuropharmacology. 2003;45:1130–1150. doi: 10.1016/s0028-3908(03)00284-3. [DOI] [PubMed] [Google Scholar]
- Song XY, Zhong JH, Wang X, Zhou XF. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci. 2004;24:542–546. doi: 10.1523/JNEUROSCI.4281-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XY, Zhou FH, Zhong JH, Wu LL, Zhou XF. Knockout of p75 impairs re-myelination of injured sciatic nerve in mice. J Neurochem. 2005 doi: 10.1111/j.1471-4159.2005.03564.x. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M. The low-affinity neurotrophin receptor p75 regulates the function but not the selective survival of specific subpopulations of sensory neurons. J Neurosci. 1997;17:4398–4405. doi: 10.1523/JNEUROSCI.17-11-04398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeder V, Grim M, Kucera J, Sieber-Blum M. Neurotrophin-3 signaling in mammalian Merkel cell development. Dev Dyn. 2003;228:623–629. doi: 10.1002/dvdy.10403. [DOI] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Fujitani M, Hata K, Mimura F, Yamagishi S. Diverse functions of the p75 neurotrophin receptor. Anat Sci Int. 2005a;80:37–41. doi: 10.1111/j.1447-073x.2005.00095.x. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Fujitani M, Yamagishi S, Hata K, Mimura F. Multiple signals regulate axon regeneration through the nogo receptor complex. Mol Neurobiol. 2005b;32:105–111. doi: 10.1385/MN:32:2:105. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Yee C, Bartel DL, Finger TE. Effects of glossopharyngeal nerve section on the expression of neurotrophins and their receptors in lingual taste buds of adult mice. J Comp Neurol. 2005;490:371–390. doi: 10.1002/cne.20670. [DOI] [PubMed] [Google Scholar]
- Zhang C, Brandemihl A, Lau D, Lawton A, Oakley B. BDNF is required for the normal development of taste neurons in vivo. Neuroreport. 1997;8:1013–1017. doi: 10.1097/00001756-199703030-00039. [DOI] [PubMed] [Google Scholar]