Abstract
Because licking and grooming behavior of dams with pups can influence some behaviors of pups when they are adults, we tested if licking and grooming scores in a maternal separation protocol correlated with cocaine or ethanol self-administration in the pups as adults. The protocol produced litters that were separated from dams for 0 (MS0), 15 (MS15) or 180 (MS180) min, and a nonhandled (NH) group as well. Self-administration of both drugs as shown in earlier studies was lowest in the MS15 group, highest in the NH group and intermediate in the other groups. Licking and grooming scores correlated negatively with drug intake and suggests that maternal care of pups can influence drug use in pups when they are adults.
Keywords: Cocaine, Alcohol, Vulnerability, Maternal care, Licking and grooming, Drug self-administration
1. Introduction
Maternal care in the post partum period can affect the development of neural systems and anxiety or fearful behaviors (Caldji et al., 1998, 2000; Francis et al., 2000; Huot et al., 2001; Francis et al., 2002). Moreover, it has been suggested that maternal care in the form of licking and grooming (LG) is a key feature in determining neural changes and offspring fear responses (Francis et al., 2002). Also, stress and fear are thought to be related to vulnerability for drug abuse (for example, see Kreek and LaForge, 2007; Sinha and Li, 2007; Poling et al., 2007; Kabbaj, 2006; Brady and Sinha, 2003).
We have recently examined the self-administration (SA) of alcohol (Jaworski et al., 2005) and cocaine (Moffett et al., 2006) in groups of litters subjected to variations in maternal separation and handling (MS/H). Groups of the pups were separated for 0 min (MS0), 15 min (MS15) or 180 min (MS180). These three groups provide a time course of effects. Another group, a nonhandled (NH) group, was also included as a control for handling. The separations are carried out from post natal day (PND) 2 to PND14, and the SA experiments were carried out in adulthood, at about PND 90. Both alcohol and cocaine SA differed among the 4 groups, but the pattern was the same for each drug: NH>MS180>MS0>MS 15 (Jaworski et al., 2005; Moffett et al., 2006). Moreover, the groups showed significant differences in 5HT1A and SERT levels, again with about the same pattern among the groups (Vicentic et al., 2006). Other investigators have found reasonably similar results (for example see Matthews et al., 1999; Campbell and Spear, 1999; Kosten et al., 2000; Flagel et al., 2003; Brake et al., 2004; Marin and Planeta, 2004; Kosten et al., 2004; Kikusui et al., 2005; Meaney et al., 2002; Hall et al., 1999).
The significance of these findings lies partly in the validity of the MS/H procedure as a model of vulnerability to human drug abuse. Human drug abuse is characterized by, among other things: 1) multidrug use, and 2) comorbidity with other psychiatric disorders. The finding that SA of cocaine, a stimulant, or ethanol, a depressant, are influenced similarly in these groups of animals suggest that the model has at least some validity for relating to human multidrug abuse. Moreover, the changes in 5HT systems, which can be related to changes in psychiatric disorders, suggest that there is a correlate to comorbidity in this model. Thus the model of maternal separation with variations in handling (MS/H) likely has utility as a model of human drug abuse vulnerability. Because LG in the perinatal period is known to influence behaviors in adulthood (Caldji et al., 1998; Francis et al., 1999; Liu et al., 1997; Menard et al., 2004; Szyf et al., 2005), we have examined the frequency of LG in these experimental groups and correlated this to cocaine and ethanol SA in similar experimental groups.
2. Methods
Experiments in this study were approved by the Emory Institutional review committee. Thirty, timed, pregnant Long Evans (Charles River Laboratories) female rats were received in the animal facility at gestation days 12-13. They were housed singly in static cages in temperature and humidity controlled rooms, maintained on a 12 h light-dark cycle with lights on at 7 AM. Food and water was available ad libitum. At birth, 6-7 litters were randomly assigned to one of the four treatment groups described below, and the separations carried out from PND2 to PND14. In any given experiment, to minimize litter effects, 1-2 male pups from each of 6-7 litters per group were used in the SA studies.
The four treatment groups include litters that were separated from their dams for 0 min (referred to as MS0), 15 min (MS15) or 180 min (MS180). Another group used as a control for handling was the NH group which was handled only once during the 2 week period for a mandatory bedding change. These procedures are more fully described in Jaworski et al. (2005). Cocaine self-administration (SA) was carried out as described by Moffett et al. (2006), and some of the data is shown here with permission. EtOH preference among the groups has been published as well (Jaworski et al., 2005) and parts are reproduced here with permission.
Licking and Grooming (LG) was examined each afternoon (1-2PM) after the separations were carried out in the morning (10 AM-12 noon). LG was also scored at 6 AM, but no differences were found. Each dam was scored every 4 min, over a 1 h period. The total number of LG observations were tabulated for each dam within the four MS/H groups.
Data were analyzed using the SPLUS program and the Pearson’s product moment correlations coefficient method for the correlations.
3. Results
Licking and grooming scores (1-2 PM) were tabulated for each of 6-7 dams in each MS/H group. Ethanol preference scores are known for each of 15 pups when they were adults (Jaworski et al., 2005). Cocaine infusion scores are also shown for each of 7-9 pups when they were adults (Moffett et al., 2006). The means and errors of the ethanol and cocaine data were published previously in graph form (Jaworski et al., 2005; Moffett et al., 2006).
When the LG scores were tested for significant differences among the four MS groups by a one way ANOVA, an overall P value of <0.0001 was found [F(3,22) = 8.764]. The average values plus or minus the SEMs are given in Fig. 1. Using Tukey’s multiple comparison test, values for MS15 vs MS180, MS180 vs MS0, and NH vs MS0 were not significantly different. However, MS15 vs NH, MS15 vs MS0, and MS180 vs NH were all different at the P<0.01 or P<0.05 level. NH had the lowest LG scores and MS15 had the highest. Scores for MS15 and MS180 groups were similar and not significantly different. Scores for the NH and MS0 groups had similar values and were not significantly different.
Fig. 1.
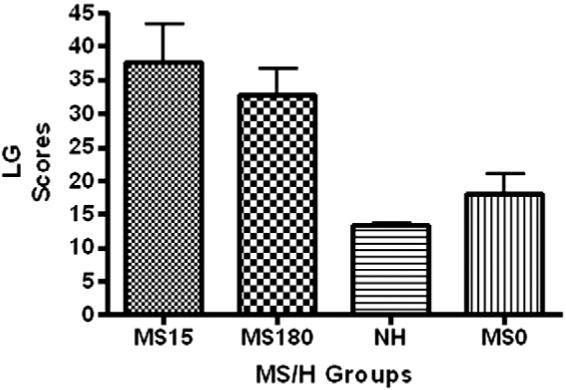
Licking and Grooming (LG) scores for dams in the four MS/H groups. Significant differences were found for MS15 vs NH, MS15 vs MS0 and for MS180 vs NH. See text for details and significant differences.
The goal is to see if there is a correlation between drug SA in pups as adults and LG activity in dams. However, the LG scores and SA scores were all from different groups of animals. Therefore multiple correlations between individual scores and averages of scores were carried out.
To test if a correlation existed between LG activity and ethanol preference, individual LG scores from the dams were correlated to the average ethanol preference scores from each MS/H group (Fig. 2 bottom), and the average LG score per group was correlated to individual scores of ethanol preference (Fig. 2, top). Fig. 2 shows a significant correlation in both cases with P values of 0.0002 and 0.0007 respectively. The correlations were negative suggesting that higher levels of licking and grooming are associated with lower drug intake.
Fig. 2.
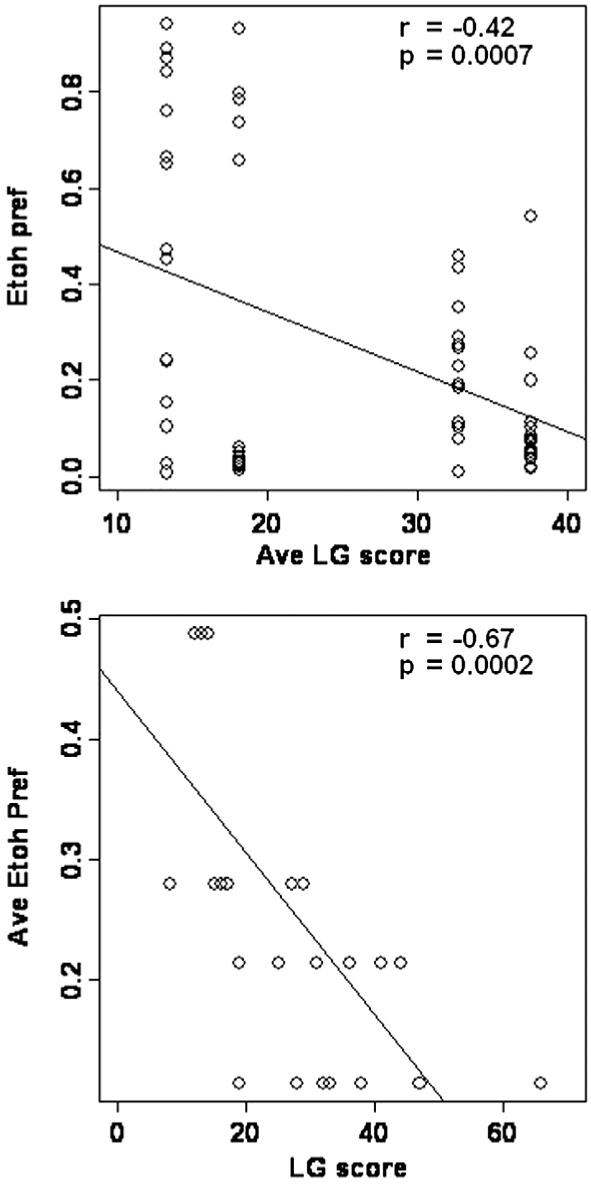
Correlations between licking and grooming (LG) activity and ethanol (EtOH) self-administration calculated as preference (Pref). Pref data are from Jaworski et al. (2005). See Text for more details.
To test for a correlation between LG activity and cocaine SA, individual LG scores were correlated to the average cocaine infusions per MS/H group (Fig. 3 top), and individual cocaine infusion measurements were correlated to the average LG scores per group (Fig. 3, bottom). Again the correlations were significant and negative supporting the idea that higher levels of licking and grooming are associated with lower levels of drug intake.
Fig. 3.

Correlations between licking and grooming (LG) activity and cocaine (Coc) self-administration shown as number of infusions of drug (Inf). Inf data are from Moffett et al. (2006). See text for more details.
4. Discussion
After MS/H separations and handlings in the morning, maternal licking and grooming scores in the early afternoon (1-2 PM) differed among the MS/H groups. The NH group had the lowest LG scores while the MS15s had the highest. Because the NH groups consistently have the highest drug intake, and the MS15s have the lowest, the LG data suggest an inverse relationship between LG and drug intake. Given that LG is a hallmark of maternal care in rats, these findings are compatible with a variety of reports suggesting that maternal care can alter the vulnerability of offspring later in life (see Introduction).
To explore this possibility further, the LG scores in one group of dams was compared and correlated to SA measures in two different groups of pups who had undergone MS/H treatments, and who had self-administered either cocaine or alcohol respectively when they were adults. There were significant correlations between LG and cocaine SA, and between LG and ethanol SA. Moreover the correlations were negative indicating that higher levels of LG correlated with less drug intake. This seems reasonable since maternal care is thought to be a positive factor in development and in later life. Also, the r2 values for the four correlations in Figs. 1 and 2 varied from 0.17 to 0.49 suggesting that there is a significant but relatively small, no more than half, contribution of LG to drug intake. If true, then other factors in addition to LG must contribute to the vulnerability to drug abuse in the MS/H animals. These other factors could include epigenetic changes, stress reactivity alterations or yet other factors. Moreover, a correlation between LG and SA does not necessarily in and of itself indicate a causal relationship.
The finding that the NH group had the largest SA and lowest LG is intriguing. In other words, lack of handling seems to create the greatest vulnerability. But, it has been noted that these are not “normal” animals such as those in the wild, because dams usually leave the nest for foraging for periods of around 20 min (Calhoun, 1963). Thus, it may be that the NH group experiences stresses that the handled groups do not. In any case, the reason for these differences among the groups needs to be resolved in future experiments.
The factors contributing to vulnerability could be interrelated. Handling could increase LG behavior of the dams, and LG could then produce epigenetic changes that might have a variety of effects. Glucocorticoids affect drug SA at least partly (Deroche-Gamonet et al., 2003; Goeders 2002), and HPA axis responses are altered in MS/H groups and this factor could play a role here. Moreover, LG levels associate with glucocorticoid receptor expression through epigenetic mechanisms in the pups which presumably alters their responses to stress (Szyf et al., 2005). The epigenetic changes in that case are due to DNA methylation and this can be reversed (Weaver et al., 2005). It seems possible that LG, through epigenetic mechanisms, could alter the reward/reinforcement pathway, perhaps by changing aspects of DA systems. Perhaps reversal of methylation will reverse vulnerability to drug intake as well. In any case, mechanisms underlying these effects can be elucidated in further experiments.
Acknowledgements
The authors acknowledge; the support of NIH grants RR00165 and DA00418, helpful discussions with Dr Mark Moffett, and guidance from our statistical consultants, Drs James Herndon and Lakshmi Chennareddi of Yerkes and Dr Kirk Easley from Emory’s statistical support group.
References
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatr. 2003;162:1483–93. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–74. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PL, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of behavioral fearfulness in adulthood in the rat. Proc Natl Acad Sci. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Calhoun J. U.S. Public Health Service. U.S. Government Printing Office; Washington DC: 1963. The ecology and sociology of the Norway rat. Publication No. 1008. [Google Scholar]
- Campbell J, Spear LP. Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology (Berl) 1999;143:183–9. doi: 10.1007/s002130050934. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, et al. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci. 2003;23:4785–90. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Vazquez DM, Robinson TE. Manipulations during the second, but not the first, week of life increase susceptibility to cocaine self-administration in female rats. Neuropsychopharmacology. 2003;28:1741–51. doi: 10.1038/sj.npp.1300228. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio, Liu D, Meaney MJ. Nongenomic transmission across generations in maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behavior are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000 Dec;12(12):1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–3. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–73. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets. 2006 Oct;5(5):513–20. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Faccidomo S, Miczek KA. Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology (Berl) 2005;178:202–10. doi: 10.1007/s00213-004-1989-1. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res. 2004;151:137–49. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS. Stress responsivity, addiction, and a functional variant of the human mu-opioid receptor gene. Mol Interv. 2007 Apr;7(2):74–8. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- Liu D, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, et al. Maternal care, hippocampal glucocorticoid receptor gene expression and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;77:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Marin MT, Planeta CS. Maternal separation affects cocaine-induced locomotion and response to novelty in adolescent, but not in adult rats. Brain Res. 2004;1013:83–90. doi: 10.1016/j.brainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 1999;141:123–34. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–38. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Menard J, Champagne D, Meaney MJ. Maternal care alters behavioral and neural activity patterns in the defensive burying paradigm. Neuroscience. 2004;129:297–308. doi: 10.1016/j.neuroscience.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Moffett M, Harley J, Francis D, Sanghani S, Davis W, Kuhar M. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther. 2006;317:1210–8. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- Poling J, Pruzinsky R, Kosten TR, Gonsai K, Sofuoglu M, Gonzalez G, et al. Clinical efficacy of citalopram alone or augmented with bupropion in methadone-stabilized patients. Am J Addict. 2007 May-Jun;16(3):187–94. doi: 10.1080/10550490701375640. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007 Jan;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005 Oct-Dec;26(34):139–62. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, et al. Maternal separation alters 5-HT transporter densities and 5-HT1A receptors in rat brain. Neuroscience. 2006;140:355–65. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005 Nov 23;25(47):11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


