Abstract
Nicotine has been found to enhance learning in a variety of tasks including contextual fear conditioning. During contextual fear conditioning animals have to learn the context and associate the context with an unconditioned stimulus (footshock). As both of these types of learning co-occur during fear conditioning it is not clear whether nicotine enhances one or both of these types of learning. To tease these two forms of learning apart we made use of the context pre-exposure facilitation effect (CPFE). Acquisition of the CPFE requires that contextual and context-shock learning occurs on separate days, allowing for their individual manipulation. Nicotine (0.09 mg/kg) administered prior to contextual learning and retrieval enhanced the CPFE whereas administration prior to context-shock learning and retrieval had no effect. Thus, nicotine enhances contextual learning but not context-shock associative learning. Finally, the results are discussed in terms of a theory of how nicotine could alter hippocampal-cortical-amygdala interactions to facilitate contextual learning.
Keywords: Nicotine, Hippocampus, Fear Conditioning, Addiction, Acetylcholine, Memory
Introduction
Nicotine is known to enhance learning and memory in a variety of tasks in both animals and humans. In rodents, nicotine administration enhances learning in the water maze (Socci, Sanberg, & Arendash, 1995), novel object recognition (Puma, Deschaux, Molimard & Bizot, 1999), and contextual fear conditioning (Gould & Wehner, 1999). Of these tasks, the neurobiology of contextual fear conditioning is particularly well understood at present (Maren, 2001; Rudy, Huff & Matus-Amat 2004; Sanders, Wiltgen & Fanselow, 2003). Given this state of affairs, contextual fear conditioning has proven fruitful in delineating the mechanisms nicotine acts upon to enhance learning and memory.
During contextual fear conditioning an animal is placed into a conditioning chamber (context) and after a specified period of time administered a foot-shock unconditioned stimulus (US). When placed back into the context 24 hours later, the animal will display conditioned fear behavior, such as freezing. During the contextual fear conditioning procedure two types of learning must occur for conditioning to take place: (1) learning the context and, (2) learning the association between the context and the shock. Both of these types of learning co-occur during the usual contextual fear conditioning training session. Thus, alterations in fear conditioning due to drug administration prior to training may be due to either altered contextual learning or altered context-shock associative learning.
Nicotine has been found to enhance contextual fear conditioning via modulation of a4β2 nicotinic acetylcholine receptors (nAChRs) in the dorsal hippocampus (Davis, Kenney & Gould, 2007). This effect is lost with chronic nicotine and deficits occur during nicotine withdrawal (Davis, James, Siegel & Gould, 2005). However, it remains unknown if these varied effects of nicotine are due to alterations in contextual learning or context-shock associative learning. One way to dissociate these two forms of learning is to make use of a variation on contextual fear conditioning known as the context pre-exposure facilitation effect (CPFE) (Fanselow, 1990; Rudy et al., 2004). When an animal is placed into a context and shocked immediately or within a few seconds of placement into the context (an immediate shock), they will show little freezing when placed back into the context 24 hours later (Fanselow, 1990). However, if a day prior to administration of the immediate shock the animal is allowed to explore the conditioning chamber for a sufficient amount of time (the context pre-exposure), the animal will show considerably more conditioning following the immediate shock (Fanselow, 1990; Frankland et al, 2004; Lattal & Abel, 2001; Rudy & O’Reilly, 1999). As contextual learning and context-shock associative learning occur on separate days, these two forms of learning can be individually manipulated.
The goal of the present study was to use the CPFE to determine which aspect of contextual fear conditioning nAChRs modulate and use the findings to develop a model of how nicotine affects the neural circuitry that underlies this task. As context learning and context-shock associative learning are thought to rely upon different neural substrates (Fanselow, 2000; Rudy et al., 2004) establishing which type of learning nicotine administration enhances will allow for a better understanding of the behavioral and neural substrates that are involved in the enhancement of learning and memory by nicotine.
Methods
Subjects
Subjects were male C57BL6/J mice aged 8 – 12 weeks. Mice were group housed (2–4 per cage) with ad libitum access to food and water and kept on a 12:12 light/dark cycle (lights on at 07.00). Training and testing occurred during the light phase. All behavioral procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Conditioning chambers used for CPFE experiments were made of clear Plexiglas (26.5 × 20.4 × 20.8 cm) and housed in sound attenuating boxes (Med Associates, St. Albans, VT). Black and white stripes were placed along the back wall and green and white stripes along one side wall of each box. The floor of each chamber comprised metal bars (0.20 cm diameter) spaced 1.0 cm apart and connected to a shock generator and scrambler (Med Associates, Model ENV-414). Ventilation fans were mounted on the sides of each box to provide background noise and air exchange. Each box was illuminated by a 4-Watt light bulb from above. Stimuli administration was controlled by a PC running LabView software.
For the experiment that made use of three different contexts to test if nicotine results in an internal representation that can enter into an association with the footshock, the following chambers were used: Context A consisted of the same chambers as above, but divided in half diagonally using a piece of Plexiglas with green stripes. The stripes along the back and side walls of the box housing the chambers were changed to solid black. Context B (27.5 × 19.5 × 16.0 cm) consisted of stainless steel side walls and Plexiglas in the front and back. Chambers were housed in sound attenuating boxes (Med Associates) with white interior walls. Metal rods comprised the floors (0.16 cm diameter, spaced 0.6 cm apart) and were connected to a shock generator and scrambler (Med Associates, Model ENV-414). Stimuli administration was controlled by a PC running Med Associates software. Context C (20.3 × 22.9 × 17.8 cm) was comprised of metal side walls, Plexiglas in the front and back and an opaque white plastic floor. Context C chambers were housed in sound attenuating boxes (Med Associates) with black interior walls and a vanilla odor was added.
CPFE Behavioral Procedures
Prior to pre-exposure, mice were handled and exposed to the transport cages for five minutes a day for two days. Transport cages were similar to the polycarbonate home cages but were of a different size and contained a mix of fresh bedding and bedding from home cages. On day 1, mice were either transported to the conditioning context and allowed to explore for 2 or 10 minutes (pre-exposed group) or transported to an adjacent room for 2 or 10 minutes and remained in the transport cages (non pre-exposed group). A subset of mice that received nicotine or saline injections prior to a 10 minute pre-exposure were scored for exploratory activity defined as crosses (all four paws across a vertical midline) and rears. On day 2 (immediate shock) all mice were transported to the conditioning chambers, and five seconds after placement in the chamber were administered a 2 second, 0.75 mA footshock. Mice remained in the chambers for 60 seconds following the immediate shock. On day 3 (testing) mice were transported to the conditioning chambers, and freezing (defined as lack of all movement aside from respiration) was assessed for one second every five seconds over three minutes. Data are presented as percent of one-second intervals mice were scored as freezing. Methods were designed based on Frankland et al (2004). Chambers were cleaned with 70% ethanol following all behavioral tasks.
Nicotine-Shock Association Test Procedures
One potential explanation for the enhancement of the CPFE by nicotine is that nicotine administration results in an internal state that enters into an association with the footshock. To test this hypothesis, on the first day of the procedure, mice were administered saline and placed into context A and trained in contextual fear conditioning as follows: Following a 148 second stimulus free period at which time baseline freezing was assessed, a 2 second, 0.35 mA footshock was administered. Following a 148 second inter-stimulus interval a second 0.35 mA footshock was administered after which mice remained in the chambers for an additional 30 seconds. Less intense footshocks were used to elicit low levels of freezing to allow for the possibility of increased freezing due to nicotine administration and to limit generalization of fear between the different contexts. On day two mice were administered nicotine and then placed into context B and trained as they were on day one. On day three mice were administered saline or nicotine and placed back into either context A or into a third novel context C and freezing was assessed over a five minute period as described above. If nicotine results in an internal state that can enter into an association with the US, then mice administered nicotine prior to testing in context A or context C should freeze more than their saline treated counterparts.
Drugs and Administration
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in physiological saline. Nicotine (0.09 mg/kg, reported as freebase; based on Gould & Higgins (2003)) or saline was administered via intraperitoneal injection 5 minutes prior to the indicated behavioral procedures at a volume of 10 ml/kg. The experimenter scoring behavior was blind to drug conditions.
Statistical Analyses
Freezing data was analyzed using 2 × 2 ANOVAs. Where appropriate Tukey post-hoc tests were used to detect significant differences between groups at the p < 0.05 level. Exploratory behavior was analyzed using independent samples t-tests. Significance was set at the p < 0.05 level.
Results
CPFE Demonstration
The goal of the first experiment was to replicate previous work in mice (Frankland et al, 2004) that found that a 10 minute, but not a 2 minute, pre-exposure to the conditioning context would be sufficient to elicit robust conditioning following the immediate shock. Mice were either pre-exposed to the context for 2 or 10 minutes or not pre-exposed to the context for an equivalent period of time (figure 1). A 2 × 2 (Exposure time X Pre-exposure condition) ANOVA revealed a significant interaction (F(1,22) = 6.84, p = 0.016): mice pre-exposed to the context for 10 minutes froze significantly more at testing than mice pre-exposed for two minutes and mice not pre-exposed.
Figure 1.

Pre-exposure to the context for ten minutes, but not two minutes, is sufficient to produce the context pre-exposure facilitation effect. *p < 0.05 compared to the 2 or 10 minute non pre-exposed and 2 minute pre-exposed groups (n = 6–7). PE – Pre-exposed, NPE – Not pre-exposed. Error bars represent SEM.
Nicotine Administration on One of Three Days
In each of three experiments, nicotine was administered either prior to the 10-minute pre-exposure, the immediate shock, or testing only. Nicotine administration prior to pre-exposure, immediate shock, or testing had no effect on freezing compared to saline treated mice (figure 2). 2 × 2 (Drug X Pre-exposure) ANOVAs revealed significant main effects due to pre-exposure condition (F(1,32) = 24.62, p < 0.001; F(1,28) = 34.98, p < 0.001; F(1,36) = 31.43, p < 0.001 for administration prior to pre-exposure, immediate shock, or testing, respectively; n’s = 8–10) and no main effect due to drug administration or interactions between drug administration and pre-exposure. In each experiment, mice pre-exposed to the context for 10 minutes the day prior to immediate shock demonstrated higher levels of freezing than those not pre-exposed, demonstrating the CPFE.
Figure 2.
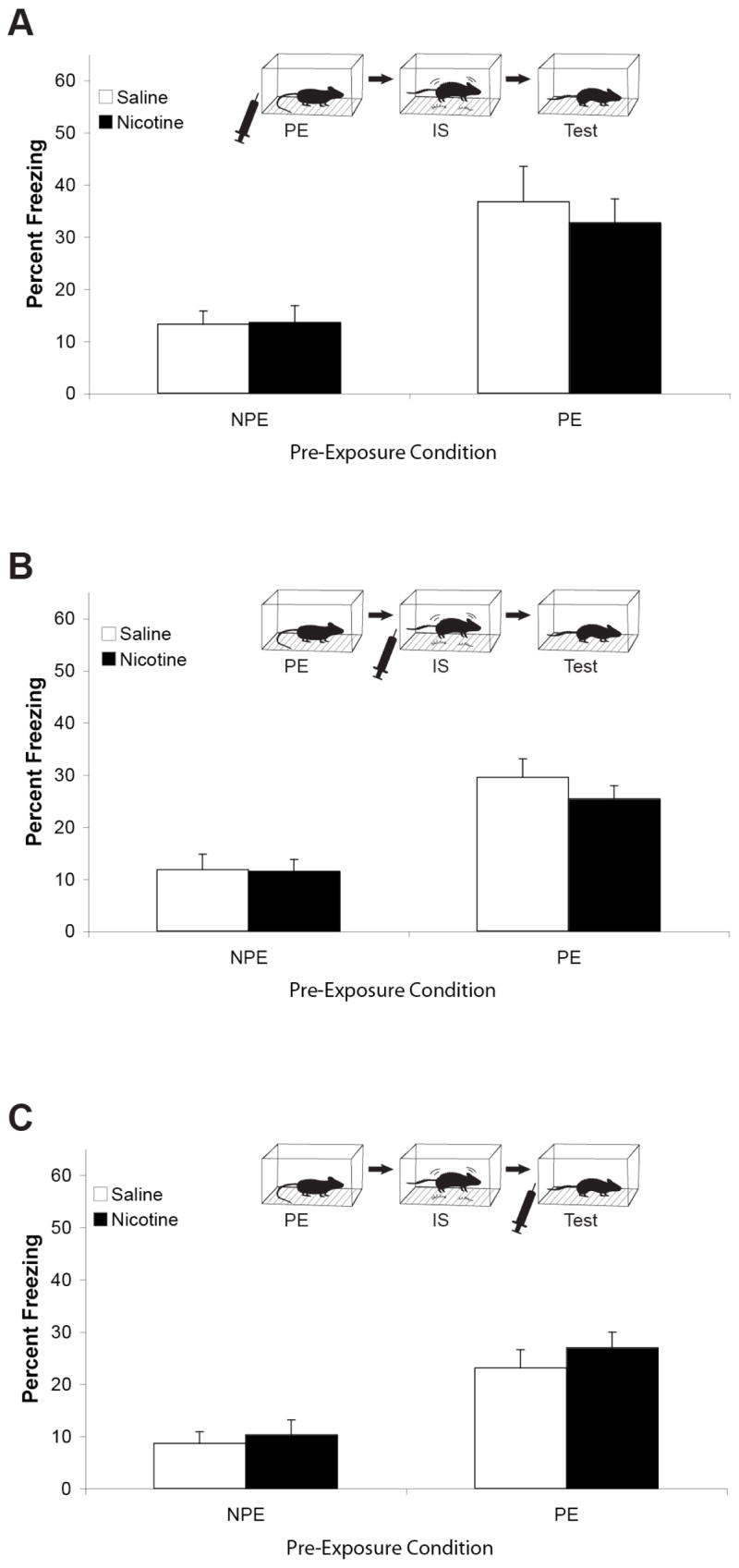
Nicotine administration prior to one of the three days that comprise the CPFE. A) Nicotine administered prior to pre-exposure only had no effect on the CPFE (n = 9). B) Nicotine administered prior to immediate shock only had no effect on the CPFE (n = 8). C) Nicotine administered prior to testing only had no effect on the CPFE (n = 10). PE – Pre-exposed, NPE –Not pre-exposed, IS – Immediate shock. Error bars represent SEM.
Nicotine Administration on Two of Three Days
In each of three experiments, nicotine was administered prior to two of the three days that comprised the CPFE procedure: prior to pre-exposure and testing, prior to immediate shock and testing, or prior to pre-exposure and immediate shock. Nicotine administration prior to immediate shock and testing or pre-exposure and immediate shock had no effect on freezing compared to saline treated mice on the CPFE (figures 3B & 3C). 2 × 2 (Drug × Pre-exposure) ANOVAs revealed a main effect due to pre-exposure in each experiment (F(1,49) = 28.56, p < 0.001; F(1,31) = 35.00, p < 0.001 respectively) and no main effect due to drug administration or interactions between drug administration and pre-exposure. Mice pre-exposed to the context for 10 minutes the day before immediate shock demonstrated higher levels of freezing than those not pre-exposed.
Figure 3.

Nicotine administration prior to two of three days that comprise the CPFE. A) Nicotine administered prior to pre-exposure and testing enhanced the CPFE (n = 9–10). B) Nicotine administered prior to immediate shock and testing had no effect on the CPFE (n = 13–14). C) Nicotine administered prior to pre-exposure and immediate shock had no effect on the CPFE (n = 8–9). * − p < 0.05 compared to both NPE groups and the PE group that received nicotine. PE –Pre-exposed, NPE – Not pre-exposed, IS – Immediate shock. Error bars represent SEM.
In contrast, mice administered nicotine prior to both the 10 minute pre-exposure and testing demonstrated higher levels of freezing than saline treated mice (figure 3A): A 2 × 2 (Drug × Pre-exposure) ANOVA revealed an interaction that was significant (F(1,33) = 12.19, p = 0.001) a main effect of pre-exposure (F(1,33) = 119.41, p < 0.001), and a main effect of drug administration (F(1,33) = 25.54, p < 0.001). Tukey post-hoc tests revealed that mice administered nicotine prior to a 10 minute pre-exposure and testing froze significantly more than the saline group that was also pre-exposed as well as both non pre-exposed groups (p < 0.05). Furthermore, the pre-exposed group that received saline froze more than both non pre-exposed groups (p < 0.05), demonstrating the expected CPFE, and the non pre-exposed groups did not differ from one another. Thus, nicotine enhances the CPFE when administered prior to both the context pre-exposure and testing.
Nicotine Administration and Exploratory Behavior
The effect of nicotine on the CPFE when administered prior to context pre-exposure and testing could potentially be explained by increased exploratory behavior during the context pre-exposure due to nicotine administration. However, if this were the case, then nicotine administration prior to only context pre-exposure would be expected to also enhance the CPFE, which was not seen. Furthermore, exploratory behavior during the 10 minute pre-exposure was not found to differ between nicotine and saline treated mice (table 1) as measured by rears (t(29) < 1), crosses (t(29) = 1.26, p > 0.05) or rears plus crosses (t(29) < 1).
Table 1.
The effect of nicotine administration on exploratory behavior during a 10 minute pre-exposure to the training context. Data presented as mean ± SEM.
| Saline (n = 15) | Nicotine (n = 16) | |
|---|---|---|
| Crosses | 23.4 ± 1.7 | 20.6 ± 1.7 |
| Rears | 19.2 ± 2.4 | 19.4 ± 2.1 |
| Crosses + Rears | 42.7 ± 3.8 | 40.0 ± 3.0 |
Nicotine-Shock Association Test
One potential explanation for the requirement that nicotine be administered prior to both pre-exposure and testing to enhance the CPFE is that, in the acquisition of the task, mice are not only forming a context-shock association, but also an association between an internal representation of nicotine and the footshock. When the memory is recalled in the presence of nicotine the nicotine-shock association summates with the context-shock association resulting in an increased fear response to the context. However, the enhancement of the CPFE does not require that nicotine is given prior to the immediate shock, thus if an internal representation of nicotine is entering into an association with the shock it must be the case that a nicotine-context association is formed during pre-exposure and nicotine administration. Then, the reactivation of the contextual memory during the immediate shock results in activation of the internal representation of nicotine that then enters into an association with the shock.
To test this hypothesis the following experiment was performed: On day one of the experiment mice were administered saline and were trained in contextual fear conditioning in context A. On day two, mice were administered nicotine and conditioned in context B, a context that generalizes little to context A (data not shown). On day three, mice were administered either saline or nicotine and tested via placement back into context A or context C. If an internal representation of nicotine can enter into an association with the footshock, this would occur on day two in context B. Then animals administered nicotine prior to placement into either context A or C during testing should show greater levels of freezing than the saline treated animals due to activation of the nicotine-shock association. The results indicate that nicotine treated mice did not differ in their freezing from their saline treated counterparts during testing when tested in either context A or context C (figure 4). A 2 × 2 (drug × test context) ANOVA revealed a significant effect of testing context (F(1,24) = 29.42, p < 0.001) such that, as would be expected, mice placed into context A froze more than those placed into context C. However, there was neither an effect due to drug administration (F(1,24) < 1) nor an interaction between drug and test context (F(1,24) < 1). Thus, the enhancement of the CPFE cannot be explained by an internal representation of nicotine becoming associated with a footshock that summates with the context-shock association.
Figure 4.

Testing whether nicotine can induce an internal state that can enter into an association with the US (footshock). Pairing nicotine with a footshock in context B and then testing in the presence of nicotine in a previously conditioned context A or a novel context C had no effect on freezing (n = 7). Error bars represent SEM.
Discussion
Nicotine administered prior to context pre-exposure and testing enhanced learning of the CPFE. In contrast, nicotine administration prior to the immediate shock and testing had no effect. Thus, nicotine enhances context learning, but not context-shock associative learning. Furthermore, nicotine had no effect when administered prior to only pre-exposure, immediate shock, or testing; nor when given prior to pre-exposure and immediate shock. Finally, nicotine administration neither affected exploratory behavior during the context pre-exposure nor resulted in an internal state that became associated with the footshock; that is the effects of nicotine did not generalize across contexts.
Contextual learning and context-shock associative learning are thought to rely on different neural substrates. Context learning is thought to depend upon the interaction between the hippocampus and the cortex whereas context-shock learning is considered to be dependent upon connections between the hippocampus and the amygdala (Fanselow, 2000; Matus-Amat, Higgins, Barrientos & Rudy, 2004; Rudy et al., 2004). During the formation of contextual memories, the hippocampus is thought to bind together various cortical regions into one configural memory trace (Rudy et al., 2004). Because nicotine did not enhance the context-shock learning that occurred on the immediate shock training day, nicotine most likely does not enhance activity in the hippocampal-amygdala pathway. The fact that nicotine alters the contextual memory suggests that nicotine is acting to strengthen connections formed during contextual learning between the hippocampus and cortex or within the hippocampus itself. This is supported by previous work showing that nicotine acts directly in the hippocampus, and not the cortex or amygdala, to enhance contextual fear conditioning (Davis et al, 2007).
Similar to results using the contextual fear conditioning paradigm (Gould & Wehner, 1999; Gould & Higgins, 2003), nicotine was required prior to both acquisition and retrieval of the context memory for enhancement of the CPFE to be observed. The effect is not state dependent, as state-dependent theory would hypothesize that there should be a deficit if the animal is tested in a different state than they were trained in (Overton, 1991). This result was not seen here or in previous studies (Gould & Wehner, 1999; Gould & Higgins, 2003), and nicotine has been shown to have little to no state dependent properties (Bevins, Penrod & Reichel, 2007).
In an effort to understand the requirement for the nicotine at both acquisition and retrieval to see the enhancement of both contextual fear conditioning and the CPFE, we have drawn upon a model of hippocampal-cortical-amygdala function based on the work of Rudy and colleagues (2004) and Fanselow (1999, 2000) (figure 5A). Briefly, the hippocampus performs two functions during the formation of a context-shock association: (1) the hippocampus acts to bind together disparate sensory cortical regions into a configural association of the context that then enters into an association with the footshock via hippocampal connections to the amygdala, and (2) the hippocampus inhibits the connections between cortical regions and the amygdala thereby preventing the strengthening of these associations (figure 5A, see Rudy et al, 2004 for discussion of the experimental evidence supporting this model). Over time, however, cortical contribution to activation of the contextual memory may increase as the role of the hippocampus decreases. Indeed, the cortical-amygdala pathway is thought to support fear conditioning in the absence of the hippocampus (Rudy et al, 2004; Sanders et al, 2003) and following long-term consolidation of the memory (Dudai, 2004; Frankland & Bontempi, 2005; Kim & Fanselow, 1992).
Figure 5.

A model of hippocampal-cortical-amygdala interaction hypothesized to underlie the enhancement of contextual fear conditioning by nicotine. C1,2,3 represent different cortical regions; A is the amygdala; red lines represent inhibitory connections; green arrows represent strengthened connections formed/exist at each stage; glowing green indicates connections further strengthened due to nicotine administration. A) The connections between hippocampus-cortex-amygdala thought to be important for contextual fear conditioning. Both the hippocampus and cortex are able to support contextual fear conditioning using configural and feature strategies respectively (Rudy et al, 2004). Further, the hippocampus has two functions in this model: (1) to bind together disparate cortical regions into a configural representation of the context that can then enter into an association with the shock via connections to the amygdala (double headed black arrows), and (2) to inhibit the connections between the cortex and amygdala (red lines). B) Two mechanisms by which nicotine is hypothesized to alter hippocampal-cortical-amygdala connectivity: (i) by strengthening hippocampal-cortical connections and (ii) by disinhibiting cortical-amygdala connections. C) The mechanisms hypothesized to underlie the enhancement of the CPFE by nicotine. Nicotine at pre-exposure enhances hippocampal-cortical associative strength that normally forms at this stage (PE + Nicotine). During the immediate shock the context-shock association is formed by a strengthening of hippocampal-amygdala connectivity. During testing in the absence of nicotine only the hippocampal-amygdala connection drives the conditioned response to the context. Nicotine administration at testing activates the previously strengthened hippocampal-cortical pathway and disinhibits the cortical-amygdala connections resulting in an increased conditioned response via greater amygdala activation.
Based on the findings in the present study that nicotine enhances the contextual learning but does not enhance the context-shock association, which suggests that nicotine may alter hippocampal-cortical activity but not hippocampal-amygdala activity, and work from the laboratories of Fanselow and Rudy (Fanselow, 1999, 2000; Rudy et al, 2004), we hypothesize that: (i) during contextual learning nicotine enhances excitatory hippocampal-cortical interactions, and (ii) during recall nicotine decreases the ability of the hippocampus to inhibit cortical-amygdala connections while again enhancing excitatory hippocampal-cortical activity (figure 5B). Thus, with nicotine on board, there is a greater cortical contribution to the contextual fear memory via changes in hippocampal activity. One prediction that follows is that over time as the memory consolidates and the hippocampus becomes less important while the cortex becomes more important, nicotine administration at recall would not be necessary to see the enhanced contextual fear memory. This, in fact, has been shown; nicotine administration was required at training and testing to see enhanced contextual fear conditioning 24 hours after training but not when reassessed one week later (Gould and Higgins, 2003).
Using these findings, we can hypothesize how nicotine enhances the CPFE when given prior to pre-exposure and testing (figure 5C). Nicotine administration at pre-exposure leads to an enhancement of the hippocampal-cortical connections (figure 5C, left). During the immediate shock, only the hippocampal-amygdala connections are strengthened forming the usual context-shock association (figure 5C, middle). On testing day in the absence of nicotine, hippocampal-amygdala connections drive recall of the context-shock association (figure 5C, top right) and the hippocampus inhibits the cortical-amygdala pathway as proposed by Rudy et al (2004). However, nicotine administered on testing day disinhibits the cortical-amygdala pathway and allows the hippocampal-cortical connection, previously strengthened during contextual learning, to activate the circuitry involved in the recall of the contextual fear-associated memory (figure 5C, bottom right). The hippocampal-cortical activation of the amygdala summates with the direct hippocampal activation of the amygdala resulting in a greater conditioned response.
Our model is also capable of explaining why nicotine administration prior to pre-exposure only, immediate shock only, or testing only does not result in an enhanced memory. Nicotine given prior to pre-exposure only has no effect, as nicotine must be given during testing day to either disinhibit cortical-amygdala connections or further enhance hippocampal-cortical interactions. Administration of nicotine prior to the immediate shock only has no effect as nicotine is not hypothesized to alter the hippocampal-amygdala connectivity that underlies the usual context-shock association. Nicotine at testing only has no effect as nicotine administration at the contextual learning stage (i.e., pre-exposure) is necessary to enhance hippocampal-cortical excitatory interactions and without this prior enhancement, hippocampal-cortical activity is not strong enough to drive enhanced expression of contextual fear conditioning at this period of memory recall.
Finally, our model can also account for why a state-dependent effect is not seen in the present study, as nicotine is not thought to affect the hippocampal-amygdala association that underlies the usual CPFE. Instead, nicotine administration results in the activation of circuits that are not necessary for this memory to form and be expressed 24 hours after acquisition. In other words, nicotine is modulating the learning by pulling in areas that are not critically involved in learning and expression of the memory at this early stage and thus it is not altering processes that are critically involved in this stage of contextual learning.
The action of nicotine at the systems level described above may be supported by a variety of mechanisms at the neurobiological level, as nAChRs are located throughout the hippocampus (Séguéla, Wadiche, Dineley-Miller, Dani & Patrick, 1993; Wada et al, 1989). nAChRs have been localized to pre-synaptic nerve terminals and can indirectly increase neurotransmitter release onto hippocampal pyramidal cells (McKay, Placzek & Dani, 2007; Wonnacott, 1997). Via this pre-synaptic mechanism, nicotine may act to enhance signaling that occurs during normal contextual learning, thereby leading to memories that are stronger than usual. Alternatively, nAChRs have also been found to act post-synaptically (Ji, Lape & Dani, 2001; McKay et al, 2007). The post-synaptic action of nicotine may lead to a reduction in the threshold required for long-term potentiation (LTP) induction or to an increase in the magnitude of LTP that may underlie the formation of the memory (Fujii, Ji, Morita & Sumikawa, 1999; Ji et al, 2001; Welsby, Rowan & Anwyl, 2006).
In addition to potentially altering plasticity via excitatory processes, nicotine has also been found to alter plasticity by modulating inhibitory mechanisms that may lead to disinhibition theorized to be important for the enhancing effect of nicotine on learning and memory. Within the hippocampus, nAChRs have been found to mediate the activation of GABAergic interneurons (Alkondon & Albuquerque, 2001; McKay et al., 2007). Nicotine is known to differentially activate interneurons of the various hippocampal and cortical layers (Alkondon & Albuquerque, 2001), and distinct interneurons express different combinations of nAChR subtypes (Alkondon & Albuquerque, 2004). As each nAChR subtype has unique functional characteristics, such as desensitization rate and affinity for nicotine (Alkondon & Albuquerque, 1993), nicotine administration may differentially alter the flow of contextual information within the hippocampus and between the hippocampus and cortex depending upon the nAChR subunits involved. Indeed, the effects of nicotine on hippocampal synaptic plasticity are complex, as nicotine can act to both inhibit or disinhibit pyramidal neuron activity, and lead to LTP or long term depression depending on the timing of neuronal activity and the activation of nAChRs (Ji et al., 2001; McKay et al, 2007).
In all, the present study demonstrates that nicotine enhances contextual fear memories by altering context learning, not context-shock associative learning. Furthermore, we suggest a hypothesis as to why nicotine must be present during both contextual learning and recall to enhance the CPFE. Our findings suggest that contextual memories formed during smoking may be strengthened. If such strengthened contextual memories enter into associations with the reinforcing effects of nicotine, as has been found in rodents (Fudala, Teoh & Iwamoto, 1985; Walters, Brown, Changeux, Martin & Damaj, 2006; Wilkinson & Bevins, 2008), then the enhanced learning may make quitting that much more difficult. In support, environmental cues can be conditioned to smoking (Lazev, Herzog, Brandon, 1999) and contextual cues that predicted smoking elicited greater cravings (Thewissen, van den Hout, Havermans, and Jansen, 2005).
Acknowledgments
We thank Karen Coletti for excellent assistance with the figures. This research was supported by the National Institute on Drug Abuse (NIDA) grant DA017949 (T.J.G.). J.W.K. was supported by NIH-NIDA training grant DA07237.
References
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. Journal of Neurophysiology. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Progress in Brain Research. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal tracking with rats. Behavioural Brain Research. 2007;177:134–141. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of Neuroscience. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nAChR involvement in the enhancing effect of acute nicotine on contextual fear conditioning. The Journal of Neuroscience. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18:264–270. [Google Scholar]
- Fanselow MS. Learning theory and neuropsychology: configuring their disparate elements in the hippocampus. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:275–283. [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nature Reviews Neuroscience. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. Consolidation of CS and US representations in associative fear conditioning. Hippocampus. 2004;14:557–569. doi: 10.1002/hipo.10208. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacology Biochemistry and Behavior. 1985;22:237–241. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Research. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiology of Learning and Memory. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behavioural Brain Research. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Abel T. An immediate-shock freezing deficit with discrete cues: a possible role for unconditioned stimulus processing mechanisms. Journal of Experimental Psychology, Animal Behavior Processes. 2001;27:394–406. [PubMed] [Google Scholar]
- Lazev AB, Herzog TA, Brandon TH. Classical conditioning of environmental cues to cigarette smoking. Experimental and Clinical Psychopharmacology. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of pavolovian fear conditioning. Annuval Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. The Journal of Neuroscience. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochemical Pharmacology. 2007;74:1120–1133. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton DA. Historical context of state dependent learning and discriminative drug effects. Behavioural Pharmacology. 1991;2:253–264. [PubMed] [Google Scholar]
- Puma C, Deschaux O, Molimard R, Bizot J. Nicotine improves memory in an object recognition task in rats. European Neuropsychopharmacology. 1999;9:323–327. doi: 10.1016/s0924-977x(99)00002-4. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and Biobehaivoral Reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. European Journal of Pharmacology. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. The Journal of Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socci DJ, Sanberg PR, Arendash GW. Nicotine enhances Morris water maze performance of young and aged rats. Neurobiology of Aging. 1995;16:857–860. doi: 10.1016/0197-4580(95)00091-r. [DOI] [PubMed] [Google Scholar]
- Thewissen R, van den Hout M, Havermans RC, Jansen A. Context-dependency of cue-elicited urge to smoke. Addiction. 2005;100:387–396. doi: 10.1111/j.1360-0443.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not the alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridizaiton histochemical study in the rat. The Journal of Comparative Neurology. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. European Journal of Neuroscience. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Bevins RA. Intravenous nicotine conditions a place preference in rats using an unbiased design. Pharmacology Biochemistry and Behavior. 2008;88:256–264. doi: 10.1016/j.pbb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends in Neurosciences. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]


