Abstract
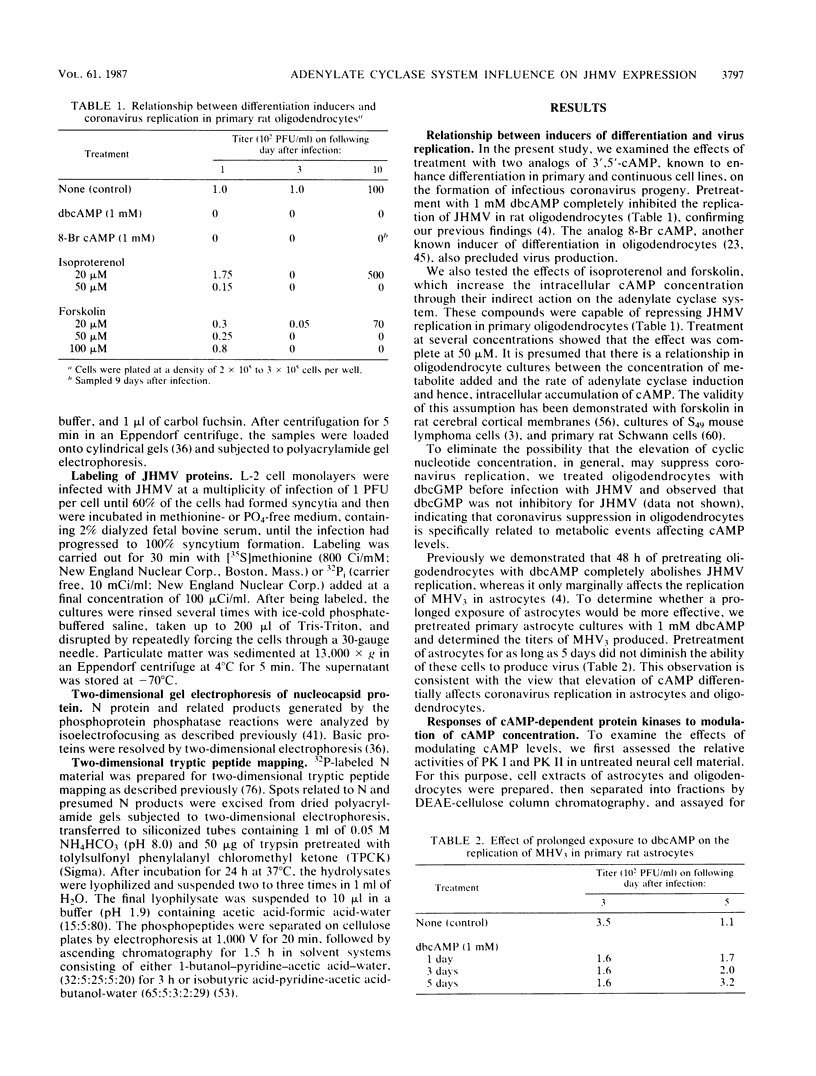
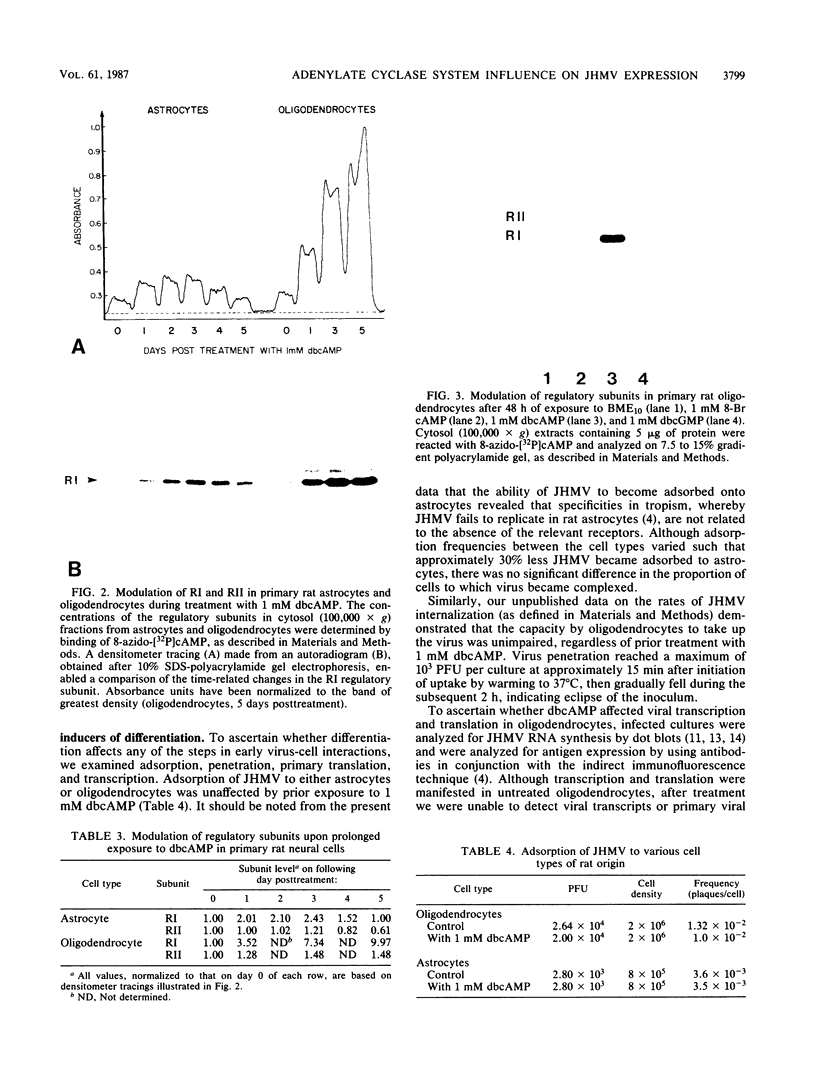
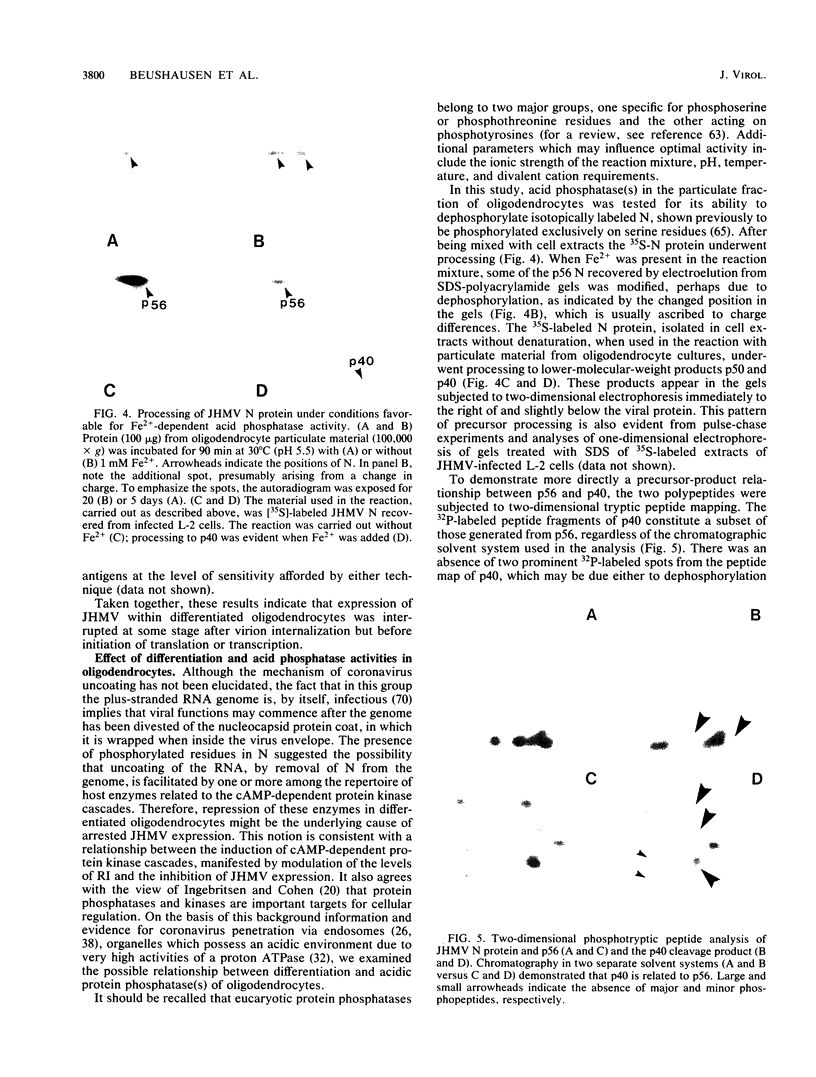
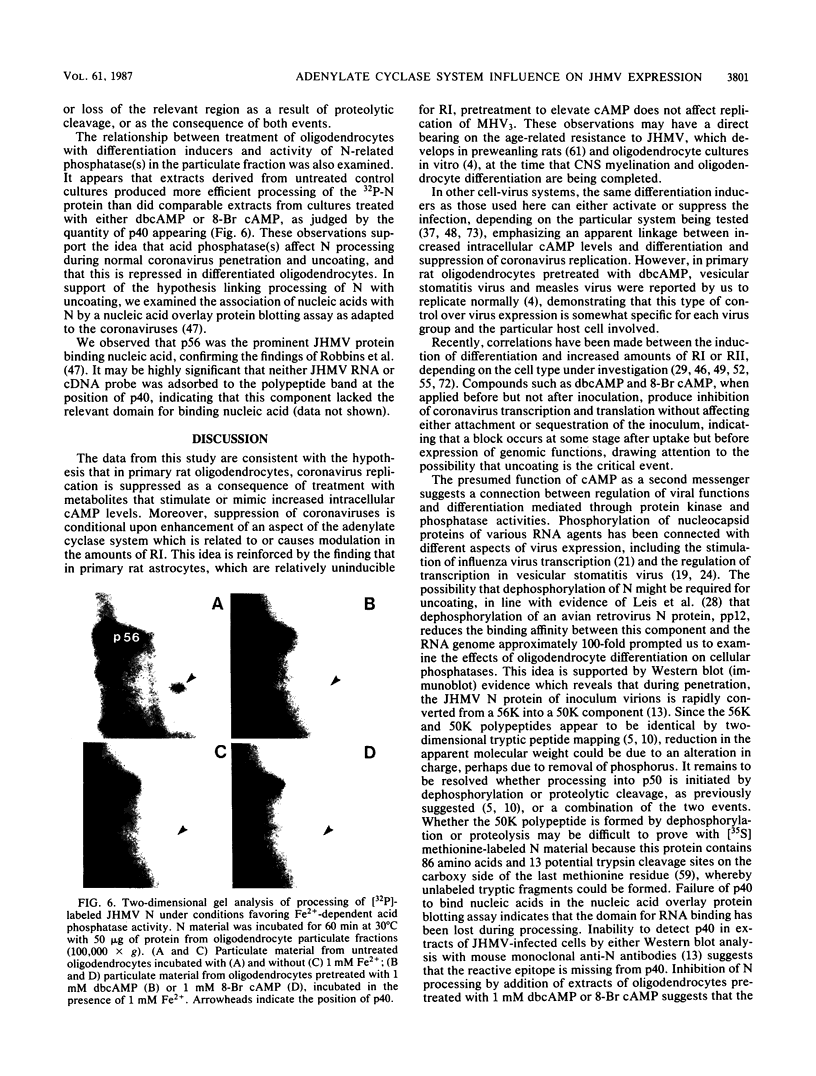
The specificity of JHM virus (JHMV) tropism for rat oligodendrocytes, as one of the primary host cells in the central nervous system, is maintained after explanation (S. Beushausen and S. Dales, Virology 141:89-101, 1985). The temporal correlation between onset of resistance to JHMV infection in vivo, completion of myelination, and maturation of the central nervous system can be simulated in vitro by inducers of oligodendrocyte differentiation (Beushausen and Dales, Virology, 1985). Stimulation of differentiation through the elevation of intracellular cyclic AMP (cAMP) levels suggests a possible connection between activation of the adenylate cyclase system and coronavirus expression. Chromatographic analysis of cAMP-dependent protein kinase activity in cytosol extracts prepared from astrocytes or oligodendrocytes revealed that both glial cell types were deficient in protein kinase I, indicating that expression of coronavirus in differentiated cells was not contingent upon the presence of protein kinase I. However, treatment with N6,2'-O-dibutyryladenosine-3',5'-cyclic monophosphate (dbcAMP) resulted in a severalfold enhancement of the free regulatory subunit (RI) in oligodendrocytes but not in astrocytes. The RII subunit in both neural cell types was relatively unaffected. Rapid increase in RI due to dbcAMP treatment was correlated with inhibition of JHMV expression. Other differentiation inducers, including 8-Br cAMP and forskolin which, by contrast, caused a decrease in detectable RI, also blocked JHMV expression. This apparent anomaly can be attributed to an increased turnover of RI due to destabilization of the molecule which occurs upon site-specific binding of the cyclic nucleotides. On the basis of these observations, we conclude that the state of oligodendrocyte differentiation manifested with the modulation of RI regulates JHMV expression. The differentiation process did not affect either virus adsorption or sequestration but appeared to inhibit the expression of viral RNA and proteins, implying that replication was inhibited at some step between penetration and initiation of genomic functions, perhaps at the stage of uncoating. We therefore examined the possibility that protein kinases and phosphatases, which influence cellular regulation during cAMP-induced differentiation, may be responsible for the phenomenon of coronavirus suppression in oligodendrocytes. Evidence was obtained indicating that normal processing of the phosphorylated nucleocapsid protein is inhibited in differentiated oligodendrocytes, consistent with the notion that JHMV replication might be arrested during uncoating.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNSTEIN T., JENSEN J. H., WAKSMAN B. H. THE DEVELOPMENT OF A NEUROTROPIC STRAIN OF MEASLES VIRUS IN HAMSTERS AND MICE. J Infect Dis. 1964 Jun;114:265–272. doi: 10.1093/infdis/114.3.265. [DOI] [PubMed] [Google Scholar]
- Barbarese E., Pfeiffer S. E. Developmental regulation of myelin basic protein in dispersed cultures. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1953–1957. doi: 10.1073/pnas.78.3.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R., Goka T. J. Adenylate cyclase activity as a function of forskolin concentration. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(1):23–29. [PubMed] [Google Scholar]
- Beushausen S., Dales S. In vivo and in vitro models of demyelinating disease. XI. Tropism and differentiation regulate the infectious process of coronaviruses in primary explants of the rat CNS. Virology. 1985 Feb;141(1):89–101. doi: 10.1016/0042-6822(85)90185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C. W., Anderson K., Leibowitz J. L. Protein synthesis in cells infected by murine hepatitis viruses JHM and A59: tryptic peptide analysis. Arch Virol. 1984;80(4):333–347. doi: 10.1007/BF01311223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody J. A., Sever J. L., Edgar A., McNew J. Measles antibody titers of multiple sclerosis patients and their siblings. Neurology. 1972 May;22(5):492–499. doi: 10.1212/wnl.22.5.492. [DOI] [PubMed] [Google Scholar]
- Cammer W., Snyder D. S., Zimmerman T. R., Jr, Farooq M., Norton W. T. Glycerol phosphate dehydrogenase, glucose-6-phosphate dehydrogenase, and lactate dehydrogenase: activities in oligodendrocytes, neurons, astrocytes, and myelin isolated from developing rat brains. J Neurochem. 1982 Feb;38(2):360–367. doi: 10.1111/j.1471-4159.1982.tb08637.x. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. Cellular synthesis and modification of murine hepatitis virus polypeptides. J Gen Virol. 1981 Jun;54(Pt 2):301–311. doi: 10.1099/0022-1317-54-2-301. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Coulter-Mackie M. B., Bradbury W. C., Dales S., Flintoff W. F., Morris V. L. In vivo and in vitro models of demyelinating diseases. IV. Isolation of Hallé measles virus-specific RNA from BGMK cells and preparation of complementary DNA. Virology. 1980 Apr 30;102(2):327–338. doi: 10.1016/0042-6822(80)90100-2. [DOI] [PubMed] [Google Scholar]
- Coulter-Mackie M., Adler R., Wilson G., Dales S. In vivo and in vitro models of demyelinating diseases. XII. Persistence and expression of corona JHM virus functions in RN2-2 Schwannoma cells during latency. Virus Res. 1985 Oct;3(3):245–261. doi: 10.1016/0168-1702(85)90049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Recurrent demyelination in chronic central nervous system infection produced by Theiler's murine encephalomyelitis virus. J Neurol Sci. 1979 Aug;42(3):391–405. doi: 10.1016/0022-510x(79)90172-2. [DOI] [PubMed] [Google Scholar]
- Dales S., Hanafusa H. Penetration and intracellular release of the genomes of avian RNA tumor viruses. Virology. 1972 Nov;50(2):440–458. doi: 10.1016/0042-6822(72)90396-0. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Corbin J. D. Regulatory mechanisms in the control of protein kinases. CRC Crit Rev Biochem. 1982 Feb;12(2):133–186. doi: 10.3109/10409238209108705. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Morgan E. M., Kingsbury D. W. Site-specific phosphorylation regulates the transcriptive activity of vesicular stomatitis virus NS protein. J Virol. 1982 Jul;43(1):104–112. doi: 10.1128/jvi.43.1.104-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Kamata T., Watanabe Y. Role for nucleocapsid protein phosphorylation in the transcription of influenza virus genome. Nature. 1977 Jun 2;267(5610):460–462. doi: 10.1038/267460a0. [DOI] [PubMed] [Google Scholar]
- Kim S. U., Moretto G., Shin D. H., Lee V. M. Modulation of antigenic expression in cultured adult human oligodendrocytes by derivatives of adenosine 3',5'-cyclic monophosphate. J Neurol Sci. 1985 May-Jun;69(1-2):81–91. doi: 10.1016/0022-510x(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Krzystyniak K., Dupuy J. M. Entry of mouse hepatitis virus 3 into cells. J Gen Virol. 1984 Jan;65(Pt 1):227–231. doi: 10.1099/0022-1317-65-1-227. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leis J., Johnson S., Collins L. S., Traugh J. A. Effects of phosphorylation of avian retrovirus nucleocapsid protein pp12 on binding of viral RNA. J Biol Chem. 1984 Jun 25;259(12):7726–7732. [PubMed] [Google Scholar]
- Lucas A., Flintoff W., Anderson R., Percy D., Coulter M., Dales S. In vivo and in vitro models of demyelinating diseases: tropism of the JHM strain of murine hepatitis virus for cells of glial origin. Cell. 1977 Oct;12(2):553–560. doi: 10.1016/0092-8674(77)90131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler F., Lohmann S. M., Walckhoff B., Walter U., Hamprecht B. Selective increase of R-I subunit of cyclic AMP-dependent protein kinase in glia-rich primary cultures upon treatment with dibutyryl cyclic AMP. Brain Res. 1985 Oct 7;344(2):322–328. doi: 10.1016/0006-8993(85)90810-8. [DOI] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Alpah-adrenergic receptor modulation of beta-adrenergic, adenosine and prostaglandin E1 increased adenosine 3':5'-cyclic monophosphate levels in primary cultures of glia. J Cyclic Nucleotide Res. 1978 Feb;4(1):15–26. [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris F. A. Cyclic AMP induction of the myelin enzyme 2',3'-cyclic nucleotide 3'-phosphohydrolase in rat oligodendrocytes. J Neurochem. 1983 Aug;41(2):506–515. doi: 10.1111/j.1471-4159.1983.tb04768.x. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Two-dimensional polyacrylamide gel electrophoresis: an improved method for ribosomal proteins. Anal Biochem. 1974 Jan;57(1):200–210. doi: 10.1016/0003-2697(74)90065-7. [DOI] [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen L., Hilton A., Cheley S., Anderson R. Attenuation of murine coronavirus infection by ammonium chloride. Virology. 1985 Apr 30;142(2):378–388. doi: 10.1016/0042-6822(85)90345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., ter Meulen V. Early and late CNS-effects of corona virus infection in rats. Adv Exp Med Biol. 1978;100:395–409. doi: 10.1007/978-1-4684-2514-7_28. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PAIGEN K., GRIFFITHS S. K. The intracellular location of phosphoprotein phosphatase activity. J Biol Chem. 1959 Feb;234(2):299–303. [PubMed] [Google Scholar]
- PAIGEN K. The properties of particulate phosphoprotein phosphatase. J Biol Chem. 1958 Aug;233(2):388–394. [PubMed] [Google Scholar]
- Pfeiffer S. E., Barbarese E., Bhat S. Noncoordinate regulation of myelinogenic parameters in primary cultures of dissociated fetal rat brain. J Neurosci Res. 1981;6(3):369–380. doi: 10.1002/jnr.490060312. [DOI] [PubMed] [Google Scholar]
- Pleasure D., Parris J., Stern J., Grinspan J., Kim S. U. Incorporation of tritiated galactose into galactocerebroside by cultured rat oligodendrocytes: effects of cyclic adenosine 3',5'-monophosphate analogues. J Neurochem. 1986 Jan;46(1):300–302. doi: 10.1111/j.1471-4159.1986.tb12963.x. [DOI] [PubMed] [Google Scholar]
- Prashad N., Rosenberg R. N. Induction of cyclic AMP-binding proteins by dibutyryl cyclic AMP in mouse neuroblastoma cells. Biochim Biophys Acta. 1978 Apr 3;539(4):459–469. doi: 10.1016/0304-4165(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Robbins S. G., Frana M. F., McGowan J. J., Boyle J. F., Holmes K. V. RNA-binding proteins of coronavirus MHV: detection of monomeric and multimeric N protein with an RNA overlay-protein blot assay. Virology. 1986 Apr 30;150(2):402–410. doi: 10.1016/0042-6822(86)90305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins S. J., Rapp F. Inhibition of measles virus replication by cyclic AMP. Virology. 1980 Oct 30;106(2):317–326. doi: 10.1016/0042-6822(80)90255-x. [DOI] [PubMed] [Google Scholar]
- Rogers J. E., Narindrasorasak S., Cates G. A., Sanwal B. D. Regulation of protein kinase and its regulatory subunits during skeletal myogenesis. J Biol Chem. 1985 Jul 5;260(13):8002–8007. [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Sato M., Hiragun A., Mitsui H. Differentiation-associated increase of cAMP-dependent type II protein kinase in a murine preadipose cell line (ST 13). Biochim Biophys Acta. 1985 Mar 21;844(3):296–305. doi: 10.1016/0167-4889(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H. Phosphorylation of simian virus 40 large T antigen: cytoplasmic and nuclear phophorylation sites differ in their metabolic stability. Virology. 1986 Apr 15;150(1):85–95. [PubMed] [Google Scholar]
- Schlosnagle D. C., Sander E. G., Bazer F. W., Roberts R. M. Requirement of an essential thiol group and ferric iron for the activity of the progesterone-induced porcine uterine purple phosphatase. J Biol Chem. 1976 Aug 10;251(15):4680–4685. [PubMed] [Google Scholar]
- Schwartz D. A., Rubin C. S. Regulation of cAMP-dependent protein kinase subunit levels in Friend erythroleukemic cells. Effects of differentiation and treatment with 8-Br-cAMP and methylisobutyl xanthine. J Biol Chem. 1983 Jan 25;258(2):777–784. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Seth P. K., Rogers J., Narindrasorasak S., Sanwal B. D. Regulation of cyclic adenosine 3':5'-monophosphate phosphodiesterases: altered pattern in transformed myoblasts. J Cell Physiol. 1983 Sep;116(3):336–344. doi: 10.1002/jcp.1041160311. [DOI] [PubMed] [Google Scholar]
- Sheahan B. J., Barrett P. N., Atkins G. J. Demyelination in mice resulting from infection with a mutant of Semliki Forest virus. Acta Neuropathol. 1981;53(2):129–136. doi: 10.1007/BF00689993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Siddell S. G. Coronavirus JHM: nucleotide sequence of the mRNA that encodes nucleocapsid protein. Nucleic Acids Res. 1983 Aug 11;11(15):5045–5054. doi: 10.1093/nar/11.15.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue G., Shuman S., Pleasure D. Schwann cell responses to cyclic AMP: proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986 Jan 1;362(1):23–32. doi: 10.1016/0006-8993(86)91394-6. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Saravani A., Dales S. In vivo and in vitro models of demyelinating disease. XVII. The infectious process in athymic rats inoculated with JHM virus. Microb Pathog. 1987 Feb;2(2):79–90. doi: 10.1016/0882-4010(87)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks J. W., Brautigan D. L. Molecular basis for substrate specificity of protein kinases and phosphatases. Int J Biochem. 1986;18(6):497–504. doi: 10.1016/0020-711x(86)90159-x. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Turnover of regulatory subunit of cyclic AMP-dependent protein kinase in S49 mouse lymphoma cells. Regulation by catalytic subunit and analogs of cyclic AMP. J Biol Chem. 1981 Nov 10;256(21):10731–10734. [PubMed] [Google Scholar]
- Stohlman S. A., Lai M. M. Phosphoproteins of murine hepatitis viruses. J Virol. 1979 Nov;32(2):672–675. doi: 10.1128/jvi.32.2.672-675.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Iwasaki Y., Koprowski H. Intracisternal virus-like particles in brain of a multiple sclerosis patient. J Neurol Sci. 1976 May;28(1):121–126. doi: 10.1016/0022-510X(76)90053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alstyne D., Paty D. W. The effect of dibutyryl cyclic AMP on restricted replication of rubella virus in rat glial cells in culture. Virology. 1983 Jan 15;124(1):173–180. doi: 10.1016/0042-6822(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Van Dinter S., Flintoff W. F. Rat glial C6 cells are defective in murine coronavirus internalization. J Gen Virol. 1987 Jun;68(Pt 6):1677–1685. doi: 10.1099/0022-1317-68-6-1677. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Dayan A. D., Allison A. C. Neuropathological effects of persistent infection of mice by mouse hepatitis virus. Infect Immun. 1975 Nov;12(5):1127–1140. doi: 10.1128/iai.12.5.1127-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter U., Costa M. R., Breakefield X. O., Greengard P. Presence of free cyclic AMP receptor protein and regulation of its level by cyclic AMP in neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3251–3255. doi: 10.1073/pnas.76.7.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Müller A., ter Meulen V. Genomic RNA of the murine coronavirus JHM. J Gen Virol. 1978 Nov;41(2):217–227. doi: 10.1099/0022-1317-41-2-217. [DOI] [PubMed] [Google Scholar]
- Weldon S. L., Mumby M. C., Taylor S. S. The regulatory subunit of neural cAMP-dependent protein kinase II represents a unique gene product. J Biol Chem. 1985 May 25;260(10):6440–6448. [PubMed] [Google Scholar]
- Wernicke J. F., Volpe J. J. Glial differentiation in dissociated cell cultures of neonatal rat brain: noncoordinate and density-dependent regulation of oligodendroglial enzymes. J Neurosci Res. 1986;15(1):39–47. doi: 10.1002/jnr.490150105. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Beushausen S., Dales S. In vivo and in vitro models of demyelinating diseases. XV. Differentiation influences the regulation of coronavirus infection in primary explants of mouse CNS. Virology. 1986 Jun;151(2):253–264. doi: 10.1016/0042-6822(86)90047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y., Yamanouchi K. Effect of papaverine treatment on replication of measles virus in human neural and nonneural cells. J Virol. 1984 May;50(2):489–496. doi: 10.1128/jvi.50.2.489-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig S. E., Singer S. J. The two components of spectrin, filamin, and the heavy chain of smooth muscle myosin show no detectable homologies to one another by two-dimensional mapping of iodinated tryptic peptides. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1147–1152. doi: 10.1016/0006-291x(79)91528-6. [DOI] [PubMed] [Google Scholar]