Abstract
In vivo MR spectroscopy (MRS) studies have shown reductions in NAA/Cr levels in patients with severe neurocognitive deficits due to AIDS dementia complex (ADC), also known as neuroAIDS. The relationship between the cellular changes within the brain during neuroAIDS and the role of NAA/Cr as a metabolic marker remains unclear. In order to clarify the relationship between NAA/Cr and disease severity we utilized the simian immunodeficiency virus (SIV)/macaque model of encephalitis. High-field proton MRS was performed on extracted metabolites from frontal cortex tissue samples of 29 rhesus macaques (6 healthy, 23 moribund with AIDS). Neuropathologic determination of encephalitis severity for each animal was completed and was found to correlate with NAA/Cr levels. Decreases in Glu/Cr and GABA/Cr may indicate that both excitatory and inhibitory neurons are affected. Highly significant correlations between NAA/Cr, Glu/Cr, and GABA/Cr were observed. These neuronal metabolites were also decreased in the absence of classical SIV encephalitis (SIVE). At any disease classification, animals inoculated with SIVmac251 were found to have lower levels of NAA/Cr than animals inoculated with SIVmac239. In considering therapy for neuroAIDS the findings here support prevention of the encephalitic process, but suggest that suppressing the formation of multinucleated giant cells alone would be insufficient to prevent neuronal injury.
Keywords: SIV, HIV, encephalitis, MRS
Human immunodeficiency virus (HIV) invades the central nervous system (CNS), causing a neurological syndrome with symptoms that may range from a mild cognitive disorder to frank dementia termed HIV-associated dementia (HAD) (1). Several in vivo proton MR spectroscopy (1HMRS) studies have shown a reduction of N-acetylaspartate (NAA), an accepted marker of neuronal dysfunction and damage, in patients with more severe neurocognitive deficits (2-5). It is unclear whether those individuals with lower NAA also had more severe HIV encephalitis, which is classically defined by the presence of perivascular macrophages/microglia, multinucleated giant cells (MNGC), and neuronal injury.
Simian immunodeficiency virus (SIV) shares similar biological properties and genetic homology with HIV-1 and HIV-2. As with HIV, the primary targets of viral replication are CD4-positive monocyte/macrophage lineage cells and lymphocytes. SIV infection in macaques causes AIDS, resulting in opportunistic infections similar to those seen in AIDS patients. In addition, like other lentiviruses including HIV, SIV invades the CNS via infected macrophages and forms MNGC, a condition termed SIV encephalitis (SIVE) (6,7). These monkeys exhibit similar in vivo MRS patterns as HIV patients with HAD (8-10). The use of the macaque model allows further examination of MRS alterations and corresponding CNS pathology, comparisons that HIV does not easily afford. Previously, we have described neuronal injury during acute SIV infection and in chronic cases without SIV giant cell encephalitis (9,10), suggesting that neuronal injury occurs even without abundant cellular infiltrates or virus in the brain. These findings also indicated that MRS NAA/creatine (NAA/Cr) is a more sensitive marker of neuronal injury and CNS disease than routine histopathology.
In this study we correlate alterations in NAA/Cr, a marker of neuronal injury and death, with severity of SIV-induced brain disease as determined by the histopathological hallmarks of encephalitis, namely, the presence of perivascular histiocytic infiltrates and MNGC in the brain parenchyma. High-resolution 1H MRS was used to analyze brain extracts from macaques divided into five groups: 1) uninfected healthy controls; 2) those chronically infected with SIV, moribund with AIDS, but lacking encephalitis, and macaques moribund with AIDS that were found to have either 3) mild, 4) moderate, or 5) severe encephalitis. Additionally, ratios of other metabolites were evaluated using MRS, especially glutamate and GABA, the major neurotransmitters of excitatory and inhibitory neuronal function, respectively.
MATERIALS AND METHODS
Animal Cohorts
The animals in this study were selected from SIV-infected rhesus macaques (Macaca mulatta) that were euthanized with terminal AIDS and submitted to the Division of Comparative Pathology at the New England Primate Research Center (NEPRC) for complete necropsy and tissue collection between 1999 and 2003. Animals were inoculated intravenously with either SIVmac251 (a dual macrophage and T-cell tropic, uncloned biological viral swarm) or SIVmac239 (a T-cell tropic virus molecularly cloned from SIVmac251). Both are pathogenic, causing immunosuppression and AIDS in rhesus macaques generally within 2 years (11,12). In addition, both viruses cause SIV giant cell encephalitis, or SIV encephalitis (SIVE), in ≈30% of infected animals (13,14). Clinical diagnosis of AIDS was based on the assessment of the clinical veterinary staff at NEPRC and included signs of marked weight loss, diarrhea, respiratory disease, or neurological signs. AIDS was confirmed based on histopathology of all organ systems and included one or more of the following AIDS-defining lesions: opportunistic infections, SIV-arteriopathy, pulmonary artery thrombosis, or giant cell disease (pneumonia, encephalitis, colitis).
The animals were housed following the standards determined by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). Investigators adhered to the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The study was approved by both Massachusetts General Hospital’s Subcommittee on Research Animal Care and the Institutional Animal Care and Use Committee of Harvard University.
Macaque Demographics
Selected animals were juveniles and adults ranging from 1.7-10.3 years of age [juvenile (weaning to sexual maturity; 3.5 years for females, 4.0 years for males), adult (sexual maturity; 3.5/4.0 to 13 years of age)]. The animals comprised 15 juveniles (range: 1.7-3.9 years, mean = 3.0 years) and 14 adults (range: 3.9-10.3 years, mean = 6.5 years). SIV-infected animals ranged from 1.7-10.3 years (mean = 4.5 years), while uninfected controls were within this range, spanning 2.3-7.3 years of age (mean = 4.4 years). Twenty animals were male and nine were female.
Tissue Collection and Neuropathology
Macaques were sacrificed by intravenous overdose injection of sodium pentobarbital. Necropsy immediately followed euthanasia. All animals infected with SIVmac251 or SIVmac239 were moribund with AIDS. Survival duration ranged from 56 to 769 days postinoculation. A standard set of formalin-fixed and frozen tissues was collected for routine histopathology. These macaques were first divided into two groups based on brain histopathology: those with giant-cell encephalitis and those without. Animals diagnosed with giant cell encephalitis were further classified as mild, moderate, or severe encephalitis based on the size and frequency of the perivascular lesions/multinucleated giant cells within the brain sections evaluated. Brain sections included cerebral cortex (frontal, parietal, temporal, occipital), basal ganglia, thalamus, hippocampus, brain stem, and cerebellum. Cases were excluded from the study that developed opportunistic CNS disease, i.e., lymphoma, progressive multifocal leukoencephalopathy (PML), and toxoplasmosis.
Adjacent brain sections were fixed in 10% neutral buffered formalin for 5-10 days, embedded in paraffin, and sectioned at 6 μm for routine histology and immunohistochemistry. Representative cases of each category (uninfected control, no giant cell encephalitis, mild, moderate, and severe encephalitis) were analyzed for astroglial activation using glial fibrillary acidic protein (GFAP) (15).
Metabolite Extraction
Frontal cortex tissue was flash-frozen in 2-methylbutane at necropsy at the NEPRC. A 50-90 mg sample was cut from the frozen tissue with a scalpel. This sample included both gray and white matter, corresponding to voxels selected during in vivo MRS experiments in other macaque studies. The sample underwent successive homogenization and centrifugation in methanol, water, and chloroform, in a Lysing Matrix D fast prep tube. This impact-resistant 2.0 mL tube containing 1.4 mm ceramic spheres, typically used for isolation of total RNA from plants and animals, was chosen to ensure thorough lysis of the tissue for metabolite extraction. The entire contents of the fast prep tube were transferred to a 15-mL centrifuge tube. After centrifugation the aqueous layer containing the metabolites was transferred into an Eppendorf tube and dried using a Savant dryer. Metabolites were redissolved in D2O to prepare the samples for MRS analysis, which was conducted while blinded to the encephalitis classification of the animals.
1H NMR Studies
High-resolution 1H MRS experiments were performed on a Bruker AVANCE 600 MHz spectrometer (Bruker Instruments, Billerica, MA) using a 5-mm broadband probe. A one-pulse experiment was used and spectral acquisition parameters included a recycle time of 20 sec, a spectral width of 7.2 kHz, 32,000 complex points, and 64 scans that were averaged. The spectral processing software Peak Research NMR Version 1/2005 (PERCH Solutions, Kuopio, Finland) was used to determine the quantities of NAA, myo-inositol (MI), total choline (total Cho; 3.19-3.22 ppm), creatine (Cr), γ-aminobutyric acid (GABA), glutamate (Glu), glutamine (Gln), N-acetylaspartylglutamate (NAAG), and glycine (Gly) in these frontal cortex samples. Since NAA is known to break down into acetate and aspartate even with the most carefully harvested and stored samples, all mention of NAA in this article will be the sum of the NAA resonance at 2.01 ppm and the acetate resonance at 1.91 ppm (16). Total Cho represents the summation of glycerophosphorylcholine (GPC), phosphorylcholine (PC), and choline (Cho) methyl resonances, since this best reflects what is measured using in vivo MRS. The metabolite values are reported as ratios with respect to the creatine peak, which is used as an internal standard. Only after completion of the spectral analysis was the pathology and classification of each tissue sample uncovered.
Statistical Analysis
For the primary hypothesis (NAA/Cr decreases with disease severity), a multivariate analysis of variance (MANOVA) was performed on all metabolite ratios across the groups. Post-hoc univariate analyses of variance (ANOVAs) for individual metabolites were carried out only if the MANOVA was significant (P < 0.05). Specific differences between pairs of groups were identified using two-tailed least squares means (LSM) Student’s t-tests only if the corresponding ANOVA was significant (P < 0.05). To confirm the authenticity of this analysis, the same procedure was repeated with the addition of age, sex, and type of inoculum as covariates to explore the possibility of their effect on the cohorts. Pearson product-moment correlation was used to determine relationships between spectroscopic markers. Bonferroni correction was applied in the case of linear regressions to adjust the significance threshold for multiple comparisons (α* = 0.017 for three pairwise correlations).
RESULTS
Neuropathology
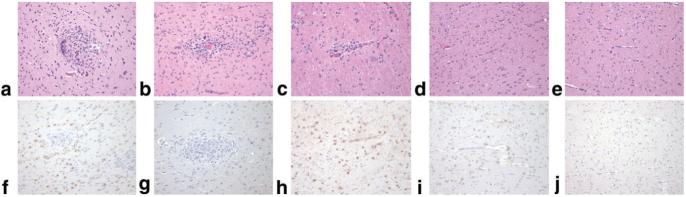
Tissues from multiple brain regions from 29 rhesus macaques were evaluated histologically to determine SIVE classification, as specified above (Table 1). Seventeen animals were classified as encephalitic, as determined by the presence of multinucleated giant cells. These 17 were further ranked as mild (n = 6), moderate (n = 4), or severe (n = 7) encephalitis based on the quantity of MNGC present in multiple regions of the brain (Fig. 1a-c). Representative sections from each of the groups (mild, moderate, and severe giant cell encephalitis) demonstrated increasing astrocytosis and neuronal injury, based on GFAP immunohistochemistry (Fig. 1f-h). Of the 23 animals moribund with SIV/AIDS, six macaques with AIDS showed no signs of giant cell encephalitis (Fig. 1d), although other mild CNS neuropathology was evident, including mild astrogliosis (Fig. 1i) and white matter spongiosis in some cases. Neuronal morphology in the frontal cortex based on cresyl violet stain was normal. The six uninfected controls lacked any signs of CNS disease or encephalitis (Fig. 1e,j).
Table 1.
Demographics of Animal Cohorts
| Animal | Sex | Agea | Survivalb | Inoculumc | Reason for Euthanasia & Other Findings | CNS Pathology |
|---|---|---|---|---|---|---|
| C1 | F | 3.9 | n.a. | n.a. | Termination of study | Healthy control |
| C2 | M | 3.7 | n.a. | n.a. | Termination of study | Healthy control |
| C3 | F | 2.5 | n.a. | n.a. | Termination of study | Healthy control |
| C4 | M | 2.3 | n.a. | n.a. | Termination of study | Healthy control |
| C5 | M | 6.9 | n.a. | n.a. | Termination of study | Healthy control |
| C6 | F | 7.3 | n.a. | n.a. | Termination of study | Healthy control |
| M1 | F | 8.5 | 70 | SIVmac251d | Wasting due to AIDS | Severe encephalitis |
| M2 | F | 9.6 | 63 | SIVmac251d | Wasting due to AIDS | Severe encephalitis |
| M3 | F | 9.6 | 56 | SIVmac251d | Wasting due to AIDS; diarrhea | Severe encephalitis |
| M4 | M | 3.0 | 157 | SIVmac251 | Facial edema; wasting due to AIDS; diarrhea | Severe encephalitis |
| M5 | M | 3.8 | 187 | SIVmac239 | Wasting due to AIDS; diarrhea; pneumonia | Severe encephalitis |
| M6 | M | 10.3 | 103 | SIVmac239 | Lethargy, poor health; CMV pneumonia | Severe encephalitis |
| M7 | M | 6.3 | 535 | SIVmac239 | Weight loss; neurologic signs | Atypical severe encephalitis |
| M8 | M | 3.9 | 124 | SIVmac251 | Wasting due to AIDS; CMV; pneumonia | Moderate encephalitis |
| M9 | F | 2.9 | 100 | SIVmac239 | Wasting; diarrhea | Moderate encephalitis |
| M10 | M | 4.5 | 67 | SIVmac251 | Wasting; anorexia; vomiting; CMV | Moderate encephalitis |
| M11 | M | 3.1 | 523 | SIVmac251 | Wasting due to AIDS; diarrhea | Moderate encephalitis |
| M12 | F | 2.6 | 176 | SIVmac251 | Enteritis; wasting due to AIDS; diarrhea | Mild encephalitis |
| M13 | M | 4.6 | 160 | SIVmac251 | Wasting due to AIDS; paresis; CMV | Mild encephalitis |
| M14 | M | 5.6 | 407 | SIVmac251 | Wasting due to AIDS; diarrhea; visual deficits | Mild encephalitis |
| M15 | M | 2.7 | 467 | SIVmac239 | Dehydration; paresis; enlarged liver | Mild encephalitis |
| M16 | M | 3.2 | 769 | SIVmac239 | Multicentric lymphoma | Mild encephalitis |
| M17 | M | 3.7 | 348 | SIVmac251 | Wasting due to AIDS; diarrhea; enteritis | Mild encephalitis |
| M18 | M | 3.4 | 293 | SIVmac251 | Severe oral disease, myocarditis | No encephalitis |
| M19 | M | 4.8 | 392 | SIVmac251 | Wasting due to AIDS; lymphoproliferative disease | No encephalitis |
| M20 | F | 4.1 | 103 | SIVmac251 | Pneumonia; oophoritis | No encephalitis |
| M21 | M | 1.7 | 173 | SIVmac239 | Wasting due to AIDS; microsporidia in liver | No encephalitis |
| M22 | M | 4.8 | 77 | SIVmac251 | Severe diarrhea; dehydration; anorexia | No encephalitis |
| M23 | M | 2.1 | 153 | SIVmac251 | Wasting due to AIDS; facial edema; anorexia | No encephalitis |
Age reported in years. The mean age was 3.0 years for juveniles and 6.5 years for adults. See Materials and Methods for explanation of juvenile and adult groupings.
Survival postinoculation reported in days.
Both SIVmac251 and 239 are known to be neurotropic and cause SIVE. N.A. indicates uninfected animals used as healthy controls.
Designates animals that were also CD8-depleted coincident with SIV inoculation.
FIG. 1.
Pathologic definition of SIV encephalitis. Representative brain histopathology (H&E stain, a-e) and immunohistochemistry of astrogliosis (GFAP, f-j) of SIV-infected rhesus macaques with giant cell encephalitis ranging from severe (a,f), and moderate (b,g), to mild (c,h), compared to SIV-infected without giant cell encephalitis (d,i) and uninfected control (e,j). Cases with severe SIV giant cell encephalitis are characterized by large perivascular lesions of macrophages and multinucleated giant cells (a) in the gray as well as white matter and marked astrogliosis with prominent GFAP immunoreactivity (f). Cases with moderate giant cell encephalitis consist of generally smaller and fewer lesions more commonly in the white matter (b) with GFAP-positive astrogliosis (g). Cases with mild giant cell encephalitis have still smaller and less frequent lesions with occasional to rare giant cells usually limited to the white matter (c) and GFAP-positive astrogliosis of the white matter (h). SIV-infected cases that do not develop giant cell encephalitis still exhibit a mild gliosis of the white matter (d) with increased GFAP immunopositivity of reactive astrocytes (i) compared to uninfected controls (e,j). Original magnification of all images is 10×.
Analysis of Metabolite Ratios with Disease Severity
Spectral analysis was performed on major brain metabolites (Fig. 2). MANOVA of all metabolite ratios across CNS disease classifications was significant (P = 0.03). Analysis of NAA/Cr for the five groups was found to be highly significant (ANOVA, P < 0.0002). Macaques in any infected group exhibited significantly lower levels of NAA/Cr than uninfected controls (Table 2). These differences in NAA/Cr levels ranged from -13% for animals with mild encephalitis (P = 0.01) to -26% for those with severe encephalitis (P = 0.000008) compared to healthy controls (Fig. 3). Furthermore, NAA/Cr levels in macaques with severe SIVE were roughly 13% lower than levels found in macaques with mild SIVE (P < 0.01) or in macaques moribund with AIDS without encephalitis (P = 0.01). Interestingly, macaques moribund with AIDS without encephalitis had similar levels of NAA/Cr as in animals with mild encephalitis (P = 0.85).
FIG. 2.
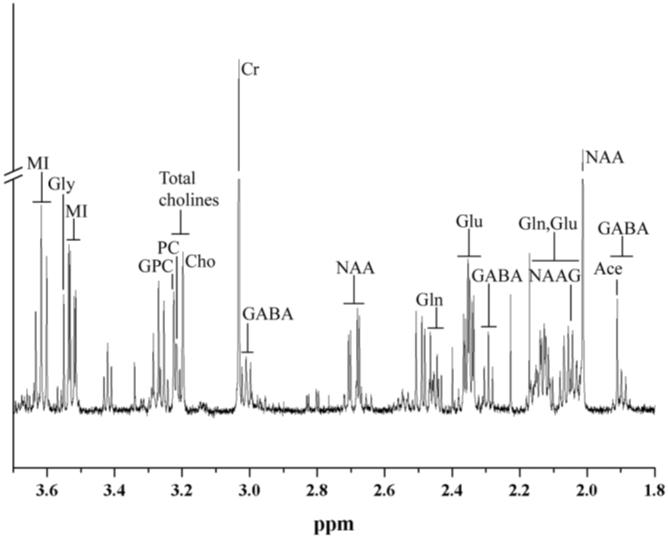
600 MHz 1H MR spectrum of a healthy macaque. Segment of a proton MR spectrum depicting extracted metabolites from the frontal cortex of a healthy rhesus macaque. Signals arising from the proton resonances of myo-inositol (MI), glycine (Gly), glycerophosporylcholine (GPC), phosphorylcholine (PC), choline (Cho), creatine (Cr), glutamine (Gln), glutamate (Glu), γ-aminobutyric acid (GABA), N-acetylaspartylglutamate (NAAG), N-acetylaspartate (NAA), and acetate (Ace) are shown. A vertical break was used, artificially lowering the intensity of the Cr and NAA peaks, so that the other metabolites could be viewed clearly. Peaks in the following regions were not included in the statistical analysis: the GABA resonances at 3.01 ppm, the NAA resonances at 2.7 ppm, and the Glu-Gln resonances between 2.0 and 2.2 ppm.
Table 2.
Summary of Least Squares Means Student’s t Tests a
| Difference of the Mean ± Standard Error of the Difference |
|||
|---|---|---|---|
| NAA/Cr | Glu/Cr | GABA/Cr | |
| Healthy control vs. macaques with AIDS (no SIVE) | -14 ± 5% | -17 ± 6% | -20 ± 7% |
| P < 0.008 | P = 0.01 | P = 0.01 | |
| Healthy control vs. mild SIVE | -13 ± 5% | -17 ± 6% | -21 ± 7% |
| P = 0.01 | P = 0.01 | P = 0.01 | |
| Healthy control vs. moderate SIVE | -21 ± 5% | -20 ± 7% | -16 ± 8% |
| P = 0.0005 | P = 0.01 | P = 0.07 | |
| Healthy control vs. severe SIVE | -26 ± 5% | -22 ± 6% | -23 ± 7% |
| P = 0.000008 | P = 0.001 | P < 0.004 | |
| AIDS (no SIVE) vs. mild SIVE | 1 ± 5% | 0 ± 6% | 0 ± 7% |
| P = 0.85 | P = 0.99 | P = 0.96 | |
| AIDS (no SIVE) vs. moderate SIVE | -7 ± 5% | -3 ± 7% | 4 ± 8% |
| P = 0.18 | P = 0.73 | P = 0.63 | |
| AIDS (no SIVE) vs. severe SIVE | -12 ± 5% | -5 ± 6% | -3 ± 7% |
| P = 0.01 | P = 0.39 | P = 0.71 | |
| Mild SIVE vs. moderate SIVE | -8 ± 5% | -2 ± 7% | 4 ± 8% |
| P = 0.13 | P = 0.74 | P = 0.60 | |
| Mild SIVE vs. severe SIVE | -13 ± 5% | -5 ± 6% | -2 ± 7% |
| P < 0.01 | P = 0.40 | P = 0.74 | |
| Moderate SIVE vs. severe SIVE | -5 ± 5% | -3 ± 7% | -7 ± 8% |
| P = 0.38 | P = 0.69 | P = 0.41 | |
LSM t tests were completed only if the corresponding ANOVA was found to be significant. All P values < 0.05 are deemed significant and are in bold for clarity.
FIG. 3.
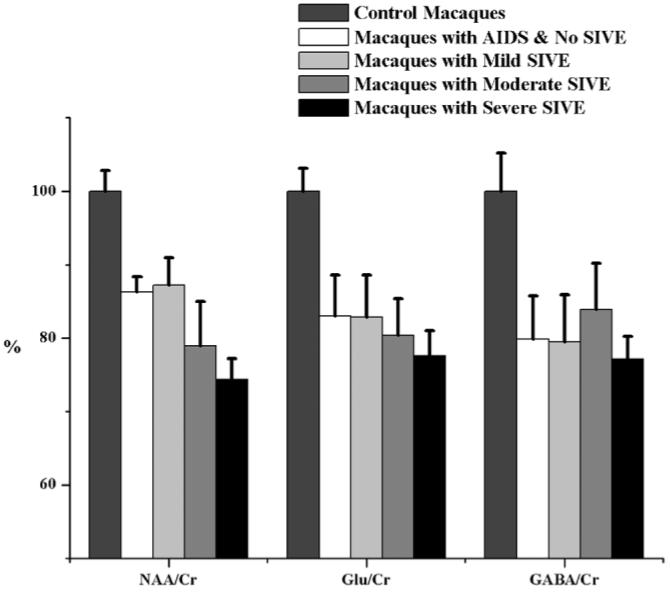
Metabolite differences across cohorts. Levels of metabolite ratios found in healthy control animals (normalized to 100%), animals moribund with AIDS without encephalitis, and animals with AIDS having varying degrees of encephalitis. ANOVA revealed significant differences in NAA/Cr, Glu/Cr, and GABA/Cr among the five cohorts. Least squares means t-tests indicated significant changes between healthy controls and all other SIV-infected groups. In the case of NAA/Cr, a significant decrease (13%) between macaques moribund with AIDS lacking encephalitis and those with severe SIVE was found. There is no statistically significant difference in any metabolite ratio between animals with mild SIVE and animals with AIDS lacking SIVE, indicating that these two classifications are more difficult to distinguish metabolically. Error bars represent standard error of the mean.
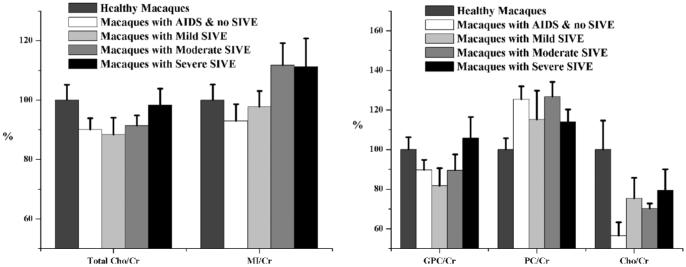
ANOVA identified specific changes in Glu/Cr (P < 0.02), assessing predominantly excitatory neurons, and GABA/Cr (P = 0.03), inhibitory neurons, within frontal cortex between CNS disease classifications and uninfected controls. Glu/Cr and GABA/Cr were decreased in all SIV-infected groups (-16 to -23%), with and without encephalitis, compared to uninfected controls (Table 2). Macaques with moderate and severe SIVE had the lowest levels of Glu/Cr. Although there was no statistically significant difference in GABA/Cr between the disease groups, there was a trend toward decreased ratios in all infected groups compared to uninfected controls. Gln/Cr, Gly/Cr, and NAAG/Cr were not altered with increasing CNS disease severity. Changes in the putative markers of glial activation (total Cho/Cr and MI/Cr), GPC/Cr, and PC/Cr were not significant (Fig. 4). Relationships between metabolite ratios were explored using linear regression. Highly significant linear relationships were discovered between NAA/Cr, GABA/Cr, and Glu/Cr (Fig. 5).
FIG. 4.
Inflammatory markers across cohorts. Mean metabolite ratios found in healthy control animals (normalized to 100%), animals moribund with AIDS without encephalitis, and animals with varying degrees of encephalitis. No significance was found in any of the total choline or myo-inositol ratios (left). Total choline was used as a factor in this model, since it best reflects what is reported using in vivo MRS. Total Cho/Cr represents the summation of the areas for glycerophosphorylcholine (GPC), phosphorylcholine (PC), and choline (Cho) (right). Error bars represent standard error of the mean.
FIG. 5.
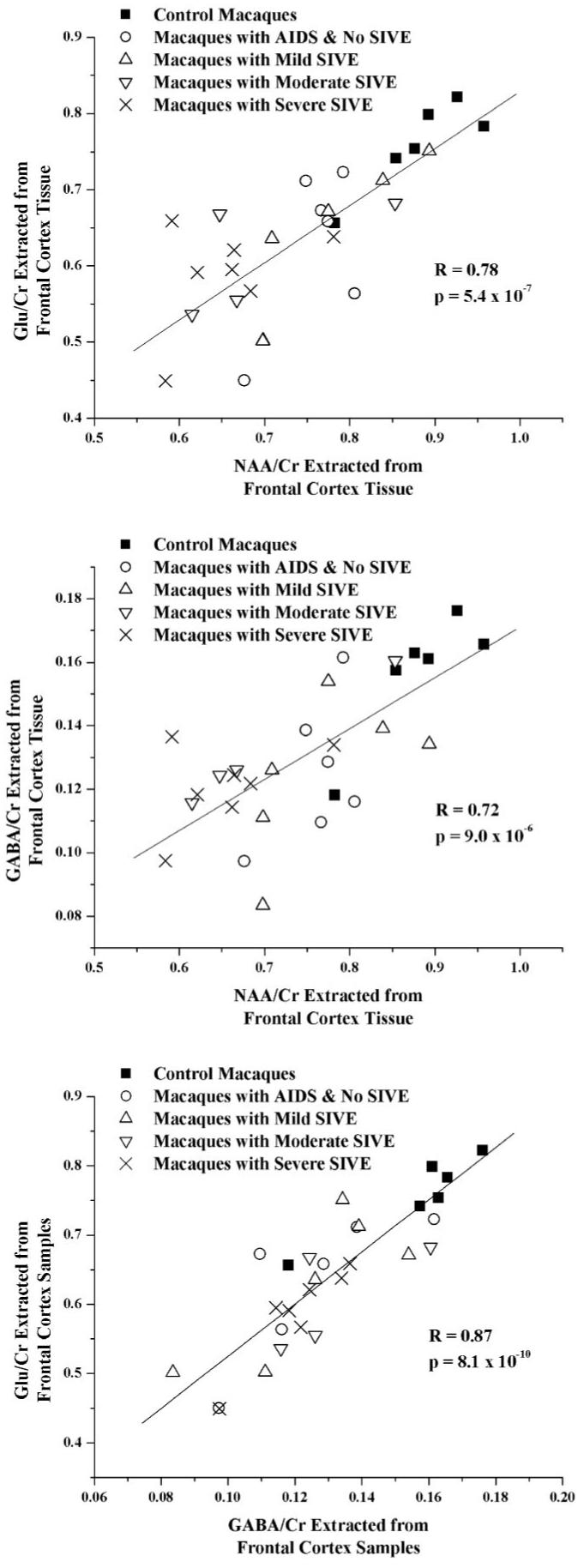
Correlations between neuronal metabolites. Highly significant correlations were observed between levels of Glu/Cr and NAA/Cr (top), GABA/Cr and NAA/Cr (middle), as well as Glu/Cr and GABA/Cr (bottom). Macaques from five cohorts are shown: healthy controls (■), macaques with AIDS and no SIVE (○), and macaques with mild SIVE (△), moderate SIVE (▽), and severe SIVE (×). Pearson correlations were significant even after Bonferroni correction for multiple comparisons.
Exploratory Analysis of Age, Sex, and Inoculum as Covariates
To verify that the differences were truly an effect of CNS disease severity, we considered the possibility of covariates significantly affecting metabolite ratios. Because CNS disease severity may be confounded with age, a second analysis was performed on all metabolite ratios across CNS disease category, age, and sex. This multivariate analysis of covariance (MANCOVA) was found to be significant (P = 0.03) in that CNS disease severity remained a statistically significant correlate of neuronal injury (decreased NAA/Cr) while neither sex nor age proved to be significant.
Finally, in order to compare the effects of viral inoculum a third analysis was conducted omitting the control animals. MANCOVA of all metabolite ratios with inoculum and CNS disease stratification as covariates (P < 0.02) indicated the role of inoculum (P < 0.005) and CNS disease severity (P = 0.056) in these animals. Analysis of the individual metabolite ratios indicated that only NAA/Cr was significantly related to the SIV inoculum, being lower in animals infected with SIVmac251 than with those infected with SIVmac239 (P < 0.0009). Effects were uniform even after controlling for the disease classification (no significant interaction). After adjusting for the type of inoculum used, NAA/Cr correlated even more closely with CNS disease severity. All pairwise differences between CNS disease stratification (those lacking SIVE vs. severe SIVE, and mild vs. severe SIVE), as reported in Table 2, remained significant. In addition, pairwise differences in NAA/Cr levels between moderate SIVE and other infected groups now approach significance when inoculum is taken into account (moderate vs. severe SIVE: P = 0.11; moderate vs. mild SIVE: P = 0.09; moderate SIVE vs. those lacking SIVE: P = 0.06).
DISCUSSION
While 1H MRS provides a reliable, noninvasive means to study the HIV-infected brain, MR studies of HIV dementia often rely on neurological (such as the ADC scale) and neuropsychological examination for evaluation of this disease (3-5). These studies have repeatedly shown that neurocognitive dysfunction correlates with metabolic changes, such as decreases in NAA/Cr and increases in Cho/Cr. However, thus far no studies have thoroughly compared a detailed metabolic analysis with terminal HIV or SIV encephalitic classifications, as this report does. Although recent reports indicate the possibility of creatine changes during the progression of this disease (17), these experiments were designed to help elucidate the substantial volume of in vivo MRS studies of HIV-infected subjects that have reported metabolite ratios. Therefore, the focus of this study was on the use of the ratio NAA/Cr as a marker of neuronal injury that reflects encephalitis severity.
Correlations between the Neuronal Marker NAA/Cr with Severity of SIVE
The initial objective of this study was to determine if neuronal injury measured by NAA/Cr correlated with histopathological severity of CNS disease classified in part by the presence and extent of MNGC. Our measurements indicated that NAA/Cr levels of the entire SIV-infected population did indeed drop as, presumably, the infiltration of monocytes/macrophages occurred and MNGC were formed. The 13-14% decreases observed in NAA/Cr levels for the mild SIVE cohort and the cohort of animals moribund with AIDS (that lack encephalitis) further support the notion that NAA/Cr may be sensitive enough to indicate injury even before pathology becomes manifest. With respect to therapy for neuroAIDS, our findings suggest that suppressing the formation of multinucleated giant cells would be insufficient to prevent neuronal injury.
We interpret a decline in NAA/Cr as reflecting neuronal metabolic dysfunction. As pointed out previously, the decline in this ratio may be due to a decline in NAA, a decrease in NAA and an increase in Cr, or simply an increase in Cr alone. The first two possibilities support our interpretation that we are observing possibly reversible neuronal metabolic dysfunction. It remains possible that only an increase in Cr is occurring, but this is not likely in light of previously published studies on the SIV/macaque model. For example, Tracey et al. (9) reported large declines in absolute NAA concentrations and no significant change in Cr in chronically infected macaques with pathologically proven SIV encephalitis. Moreover, Gonzalez et al. (10) found that in acute and chronically infected macaques a decline in NAA/Cr was observed along with quantitative neuropathologic measures of neuronal injury. Finally, in investigations by Greco et al. (18) and Lentz et al. (20) that involved both in vivo and ex vivo MRS using this model, it was shown that reversible changes in NAA/Cr best correlated with changes in the neuronal marker synaptophysin as quantified by immunohistochemistry.
In studies of human neurodegenerative diseases correlating MRS measurements with neuropathology, observations are very similar to the macaque model. For example, numerous publications have described declines in NAA/Cr by in vivo MRS in several neurodegenerative diseases such as Pick’s disease and Alzheimer’s disease. The neuropathological correlates of these in vivo observations have been reported by Cheng et al. (16,24). They reported excellent correlations between neuronal loss measured by traditional neurohistopathology methods and decreases of NAA measured by HRMAS 1H MRS in Pick’s disease (16). They also showed that in brains from proven Alzheimer’s disease patients and controls NAA was found to correlate highly with neuronal number determined by stereology, the most precise quantitative neuropathologic technique for counting neurons (24). In a review of the literature, we have not found any human studies of neurodegenerative disease or relevant animal models that have found only increases in Cr coupled with neuropathologic measurements. Taken together, the great preponderance of evidence suggests that declines in NAA or NAA/Cr are best explained by neuronal metabolic dysfunction, and that while possible, increases in Cr alone are much less likely to explain the observations.
Changes in Glu/Cr and GABA/Cr
Levels of Glu/Cr and GABA/Cr were found in all disease classifications to be lower than those of healthy control animals. Since Glu and GABA are neurotransmitters that are produced in high concentrations in excitatory and inhibitory neurons, respectively, the decline in both ratios can be interpreted as reflecting injury to both classes of neurons, although an increase in Cr could explain some of the changes. However, unlike NAA/Cr, differences in these metabolite ratios could not discriminate severity of encephalitis. Although studies have suggested that glutamate excitotoxicity plays a role in the neuronal death found in neuroAIDS (25,26), MRS may not have the sensitivity to detect increases in the micromolar concentrations of extracellular glutamate relative to the millimolar concentrations of intracellular glutamate, which is orders of magnitude greater. In fact, in vivo MRS reports on patients with ADC have shown significant decreases of Glx (the summation of glutamate and glutamine concentrations) in the frontal cortex (17). AIDS patients with PML lesions were found to have decreases in Glx within the lesion (P = 0.09) and within contralateral tissues (P = 0.02) (27).
Bossuet et al. (28) have previously described increases in glutamate levels for SIVmac251-infected macaques. However, there are important differences that preclude a direct comparison of their results to ours. Their longitudinal, serial sacrifice study was conducted across asymptomatic and symptomatic periods of SIV disease progression, while the current report is a specific exploration of terminal AIDS. In addition, their animals were from another species (cynomolgus), and the mean age of the controls was very young (13 months) compared to the infected cohort (59 months). Only six of their 14 SIV-infected animals had histologic markings of gliosis, and no statistical correlation between astrocyte activation, monocyte/macrophage infiltration, and metabolite changes was found. There also was a lack of significant changes in glutamate levels in the CSF (as determined by HPLC) between control and infected macaques. Significant elevations in picomolar concentrations of CSF glutamate have been observed in HIV subjects and correlated to dementia severity (26).
Correlations between NAA/Cr, Glu/Cr, and GABA/Cr
The highly significant correlation between NAA/Cr and Glu/Cr may be indicative of metabolic dysfunction in the NAA-glutamine cycle between neurons and astrocytes, as proposed by Petroff et al. (29) and many others before. Astrocytes contain only a small amount of the total glutamate concentration within the brain (30), and by advanced stages of neuroAIDS, astrocytes are known to have less uptake and a greater release of glutamate due to inflammatory factors (1). GABAergic neurons have very little glutamate, as it is rapidly converted to GABA, whereas the majority of glutamate is found in glutamatergic neurons (30). Thus, the glutamate and GABA ratios measured by MRS could reflect the integrity of glutamatergic and GABAergic neurons, respectively. In general terms, during the early stages of pure SIV/HIV encephalitis the pyramidal glutamatergic neurons are most affected, whereas in the later stages GABAergic and somatostatin neurons are also affected (correspondence with Dr. Eliezer Masliah). Therefore, decreases in all three ratios are to be expected at later stages of neuroAIDS. However, it is interesting to note that all three metabolite ratios are decreased even in animals that lack any signs of encephalitis.
Changes in Markers of Glial and Membrane Turnover
Cho/Cr, a marker of membrane turnover, and MI/Cr, a glial marker, are often reported as being elevated in in vivo MR spectroscopy of HIV+ subjects. The lack of change in total Cho/Cr was surprising and led us to examine the three individual metabolites that make up the choline region (Fig. 4). Interestingly, the metabolite ratio of phosphorylcholine visually appears to be the only choline derivative that is increased with SIV infection and disease severity, but this elevation was not significant. Recently, we reported inconsistencies between total Cho/Cr measures in 1H HRMAS spectra and in the extracted metabolite spectrum for the same tissues, while all other metabolites correlated very well (31). Greater error in Cho/Cr measurements using both in vivo MRS and 1H HRMAS may be caused by overlapping metabolite resonances of the in vivo 1H spectrum, including contributions from lipids and macromolecules. In vivo MRS of acute SIV infection indicated large increases in Cho/Cr levels that quickly dropped below preinfection levels within the first 30 days of infection (18). The amount of virus in the blood was significantly correlated with the degree of astrocytosis in the frontal cortex and with in vivo 1H MRS Cho/Cr (19). However, ex vivo MRS results indicated that the in vivo changes in Cho/Cr were principally due to contributions other than those of water-soluble metabolites. It is also possible that elevations in MI or Cho are being obscured by Cr elevations. The present set of experiments does not allow us to ascertain this possibility, and future studies are needed to clarify this issue.
Similarities between SIV+ Animals with Mild Encephalitis and Those without SIVE
It is also interesting to note that the levels of NAA/Cr, Glu/Cr, GABA/Cr, MI/Cr, and total Cho/Cr for animals with mild SIVE were similar to those that were likewise moribund with AIDS but lacked encephalitis. This observation indicates that these two disease classifications are more difficult to distinguish from one another metabolically. The sensitivity of our technique may be insufficient to detect small differences between them.
Effects of SIV Inoculum and Age on NAA/Cr
NAA/Cr was lower in animals infected with SIVmac251 than with those infected with SIVmac239. This is interesting since both viral strains are used in SIV research, and both are known to cause SIVE. At the nucleotide level these two inocula are closely related, with only a 2% variance (32). Westmoreland et al. (13) reported higher incidence of SIVE in animals with SIVmac251 than those with SIVmac239. One possible cause for the difference is that SIVmac239 does not initially replicate in macrophages (an immune cell capable of productive infection implicated in SIVE pathogenesis), and must undergo change in cell tropism in vivo to allow replication in these cells. Indeed, SIVmac239 has been found to induce SIVE after the development of significant viral variation that spontaneously occurs in up to 30% of animals (33). Previous work by Montgomery et al. (34) indicated that hippocampal pyramidal cell diameters in cynomolgus macaques were more reduced by SIVmac251 (-16.6%) than by SIVmac239 (-6.8%). The absence of neuronal atrophy was also accredited to low CNS viral load and subsequent viral replication due to reduced macrophage entry into the brain. These reports, combined with the data in this article, suggest that SIVmac251 may cause an earlier macrophage infiltration into the brain, and that the injury is cumulative over time, resulting in lower levels of NAA/Cr in animals over the course of infection.
Although the incidence of neurological disease has declined with the advent of antiretroviral therapy (35), the prevalence of cognitive deficits due to HIV as well as normal aging may increase as patients live longer. Consequently, age has become a more prominent topic in many HIV/MRI studies and could potentially confound the relationship between metabolites and disease progression (36-38). In particular, aging was found to possibly exacerbate changes in brain metabolites associated with inflammation in HIV patients over the age of 40, thereby increasing the likelihood of cognitive impairment. Also, HIV-infected children do not demonstrate a normal age-associated increase in NAA in frontal white matter, indicating that HIV may impact neuronal maturation (38). It was also observed that healthy children from ages 6 to 16 still have age-associated changes in both metabolite concentrations and metabolite ratios during childhood and adolescence (38-40). Due to the limited age range of the animals within the study (1.7-10.3 years old), small cohort size, and appropriate distribution of age across cohorts, we did not find a significant age effect for this model. However, this does not rule out the possibility that some metabolites in juvenile and adult animals may change slowly with age, as is seen in humans.
CONCLUSION
This study confirms our prior observations of neuronal injury in SIV-infected animals even in the absence of classic encephalitis and demonstrates that ex vivo NAA/Cr measures can be used for disease severity ranking. The most severe encephalitis was accompanied by the greatest reduction in neuronal metabolites. NAA/Cr was the only metabolic marker that directly correlated with the severity of disease classification. Our findings that glutamate and GABA ratios are also diminished may suggest injury to both excitatory and inhibitory neurons, although it is not yet clear how Cr affects the ratios. In considering therapy for neuroAIDS, the findings here support preventing or minimizing the encephalitic process, but suppressing the formation of multinucleated giant cells alone would be insufficient to fully prevent neuronal injury.
ACKNOWLEDGMENTS
The authors thank Dr. Eliezer Masliah for helpful comments about glutamatergic and GABAergic neuronal dysfunction in HIV, and Elizabeth Curran for pathology assistance, and the contribution of tissues from Drs. Desrosiers, Kaur, Lackner, Mansfield, Shannon, and Tzipori.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: RR13213 (to R.G.G.), NS050041 (to R.G.G.), NS051129 to (M.R.L.), NS34626 (to R.G.G.), RR000150 (to S.V.W.), RR00168-39 (to the NEPRC), P41 RR00995 (to MIT); Grant sponsor: National Center for Research Resources; Grant number: P41 RR14075; Grant sponsor: Mental Illness and Neuroscience Discovery (MIND) Institute.
REFERENCES
- 1.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 2.Avison MJ, Nath A, Berger JR. Understanding pathogenesis and treatment of HIV dementia: a role for magnetic resonance? Trends Neurosci. 2002;25:468–473. doi: 10.1016/s0166-2236(02)02234-8. [DOI] [PubMed] [Google Scholar]
- 3.Tracey I, Carr CA, Guimaraes AR, Worth JL, Navia BA, Gonzalez RG. Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: a proton magnetic resonance spectroscopic study. Neurology. 1996;46:783–788. doi: 10.1212/wnl.46.3.783. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, Miller EN. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999;53:782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- 6.Burudi EM, Fox HS. Simian immunodeficiency virus model of HIV-induced central nervous system dysfunction. Adv Virus Res. 2001;56:435–468. doi: 10.1016/s0065-3527(01)56035-2. [DOI] [PubMed] [Google Scholar]
- 7.Sharer LR, Baskin GB, Cho ES, Murphey-Corb M, Blumberg BM, Epstein LG. Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol. 1988;23(Suppl):S108–112. doi: 10.1002/ana.410230727. [DOI] [PubMed] [Google Scholar]
- 8.Greco JB, Sakaie KE, Aminipour S, Lee PL, Cheng LL, He J, Westmoreland S, Lackner AA, Gonzalez RG. Magnetic resonance spectroscopy: an in vivo tool for monitoring cerebral injury in SIV-infected macaques. J Med Primatol. 2002;31:228–236. doi: 10.1034/j.1600-0684.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- 9.Tracey I, Lane J, Chang I, Navia B, Lackner A, Gonzalez RG. 1H magnetic resonance spectroscopy reveals neuronal injury in a simian immunodeficiency virus macaque model. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:21–27. doi: 10.1097/00042560-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, Lee PL, Masliah E, Lackner AA. Early brain injury in the SIV-macaque model of AIDS. Aids. 2000;14:2841–2849. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- 11.Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, MacKey JJ, Schmidt DK, Chalifoux LV, King NW. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 12.King NW, Chalifoux LV, Ringler DJ, Wyand MS, Sehgal PK, Daniel MD, Letvin NL, Desrosiers RC, Blake BJ, Hunt RD. Comparative biology of natural and experimental SIVmac infection in macaque monkeys: a review. J Med Primatol. 1990;19:109–118. [PubMed] [Google Scholar]
- 13.Westmoreland SV, Halpern E, Lackner AA. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- 14.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 15.O’Neil SP, Suwyn C, Anderson DC, Niedziela G, Bradley J, Novembre FJ, Herndon JG, McClure HM. Correlation of acute humoral response with brain virus burden and survival time in pig-tailed macaques infected with the neurovirulent simian immunodeficiency virus SIVsmmFGb. Am J Pathol. 2004;164:1157–1172. doi: 10.1016/S0002-9440(10)63204-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng LL, Ma MJ, Becerra L, Ptak T, Tracey I, Lackner A, Gonzalez RG. Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997;94:6408–6413. doi: 10.1073/pnas.94.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 18.Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51:1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- 19.Kim JP, Lentz MR, Westmoreland SV, Greco JB, Ratai EM, Halpern E, Lackner AA, Masliah E, Gonzalez RG. Relationships between astrogliosis and 1H MR spectroscopic measures of brain choline/creatine and myo-inositol/creatine in a primate model. AJNR Am J Neuroradiol. 2005;26:752–759. [PMC free article] [PubMed] [Google Scholar]
- 20.Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern EF, Lackner AA, Masliah E, Gonzalez RG. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- 21.Fuller RA, Westmoreland SV, Ratai E, Greco JB, Kim JP, Lentz MR, He J, Sehgal PK, Masliah E, Halpern E, Lackner AA, Gonzalez RG. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004;5:10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez RG, Greco JB, He J, Lentz MR, O’Neil S, Pilkenton S, Ratai EM, Westmoreland SV. New insights into the neuroimmunity of SIV infection by magnetic resonance spectroscopy. J NeuroImmune Pharmacol. 2006;1:152–159. doi: 10.1007/s11481-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 24.Cheng LL, Newell K, Mallory AE, Hyman BT, Gonzalez RG. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging. 2002;20:527–533. doi: 10.1016/s0730-725x(02)00512-x. [DOI] [PubMed] [Google Scholar]
- 25.Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 26.Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, Singer E, Cornford M. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997;48:836–845. doi: 10.1212/wnl.48.4.836. [DOI] [PubMed] [Google Scholar]
- 28.Bossuet C, Vaufrey F, Conde F, Chretien F, Pichon J, Hantraye P, Le Grand R, Dormont D, Gras G. Up-regulation of glutamate concentration in the putamen and in the prefrontal cortex of asymptomatic SIV-mac251-infected macaques without major brain involvement. J Neurochem. 2004;88:928–938. doi: 10.1046/j.1471-4159.2003.02237.x. [DOI] [PubMed] [Google Scholar]
- 29.Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Neuronal and glial metabolite content of the epileptogenic human hippocampus. Ann Neurol. 2002;52:635–642. doi: 10.1002/ana.10360. [DOI] [PubMed] [Google Scholar]
- 30.Ottersen OP. Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol (Berl) 1989;180:1–15. doi: 10.1007/BF00321895. [DOI] [PubMed] [Google Scholar]
- 31.Ratai EM, Pilkenton S, Lentz MR, Greco JB, Fuller RA, Kim JP, He J, Cheng LL, Gonzalez RG. Comparisons of brain metabolites observed by HRMAS 1H NMR of intact tissue and solution 1H NMR of tissue extracts in SIV-infected macaques. NMR Biomed. 2005;18:242–251. doi: 10.1002/nbm.953. [DOI] [PubMed] [Google Scholar]
- 32.Regier DA, Desrosiers RC. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 33.Mori K, Ringler DJ, Kodama T, Desrosiers RC. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992;66:2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery MM, Wood A, Stott EJ, Sharp C, Luthert PJ. Changes in neuron size in cynomolgus macaques infected with various immunodeficiency viruses and poliovirus. Neuropathol Appl Neurobiol. 1998;24:468–475. doi: 10.1046/j.1365-2990.1998.00147.x. [DOI] [PubMed] [Google Scholar]
- 35.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 36.Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 37.Pfefferbaum A, Rosenbloom M, Sullivan EV. Alcoholism and AIDS: magnetic resonance imaging approaches for detecting interactive neuropathology. Alcohol Clin Exp Res. 2002;26:1031–1046. doi: 10.1097/01.ALC.0000021146.01778.55. [DOI] [PubMed] [Google Scholar]
- 38.Keller MA, Venkatraman TN, Thomas A, Deveikis A, LoPresti C, Hayes J, Berman N, Walot I, Padilla S, Johnston-Jones J, Ernst T, Chang L. Altered neurometabolite development in HIV-infected children: correlation with neuropsychological tests. Neurology. 2004;62:1810–1817. doi: 10.1212/01.wnl.0000125492.57419.25. [DOI] [PubMed] [Google Scholar]
- 39.Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J Magn Reson Imaging. 2002;15:137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- 40.van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990;176:509–515. doi: 10.1148/radiology.176.2.2164237. [DOI] [PubMed] [Google Scholar]