Abstract
We previously reported that squalestatin 1-mediated induction of CYP2B expression is attributable to squalene synthase inhibition and accumulation of an endogenous isoprenoid(s) that is capable of activating the constitutive androstane receptor. To determine whether squalestatin 1-mediated CYP2B induction is strictly dependent upon the biosynthesis of farnesyl pyrophosphate (FPP), the substrate for squalene synthase, the effects of alendronate, a nitrogen-containing bisphosphonate inhibitor of farnesyl diphosphate synthase, were determined on basal, squalestatin 1-inducible, and phenobarbital-inducible CYP2B expression in primary cultured rat hepatocytes. Alendronate treatment alone had no effect on CYP2B or CYP3A mRNA expression in the hepatocyte cultures, but alendronate co-treatment completely suppressed squalestatin 1-mediated CYP2B mRNA induction at concentrations (60 and 100 μM) that effectively inhibited cellular farnesyl diphosphate synthase activity, as assessed by reductions of squalestatin 1-mediated FPP accumulation, and that were not toxic to the cells, as indicated by a lack of effect on MTT activity. Alendronate co-treatment also partially suppressed phenobarbital-inducible CYP2B expression, and this suppressive effect was attenuated by additional co-treatment with the upstream pathway inhibitor, pravastatin. These findings demonstrate that squalestatin 1-mediated CYP2B induction cannot occur in the absence of FPP biosynthesis, but also indicate that one or more upstream isoprenoids, possibly isopentenyl pyrophosphate and/or dimethylallyl pyrophosphate, function to antagonize the CYP2B induction process.
Introduction
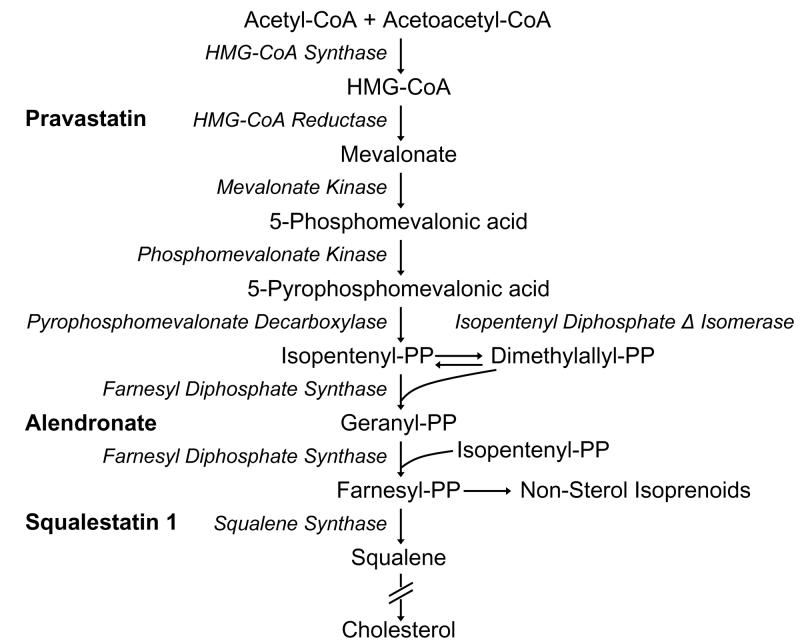
Phenobarbital and “phenobarbital-like” chemicals induce the transcription of CYP2B and other genes by activating CAR [see (Kodama and Negishi, 2006) for recent review]. We previously reported that treatment of rats or primary cultured rat hepatocytes with squalestatin 1 (also known as zaragozic acid A), an inhibitor of squalene synthase, the first committed step in cholesterol biosynthesis, caused the selective induction of CYP2B (Kocarek, et al., 1998), relative to other inducible P450s, and that this induction was attributable to squalene synthase inhibition and the accumulation of an endogenous isoprenoid(s) that is capable of activating CAR (Kocarek and Mercer-Haines, 2002) (see Fig. 1 for scheme of the isoprenoid pathway).
Fig. 1.
Isoprenoid biosynthetic pathway showing sites of inhibition by pravastatin, alendronate and squalestatin 1.
In continuation of this investigation, we sought to determine whether squalestatin 1-mediated CYP2B induction was strictly dependent upon the biosynthesis of FPP, the substrate for squalene synthase. FPP is formed through the FDPS-catalyzed condensation of one molecule of GPP with one molecule of IPP. In addition to its role as a sterol precursor, FPP is a branchpoint intermediate that is metabolized by several other enzymes, including protein farnesyl transferase, which catalyzes the farnesylation of certain proteins, such as small GTPases (Basso, et al., 2006); GGPP synthase, which catalyzes the formation of GGPP (Kavanagh, et al., 2006), which is also utilized in protein prenylation reactions (Basso, et al., 2006); trans-prenyltransferase, which leads to the biosynthesis of ubiquinone (although in mammals, GPP is this enzyme's preferred substrate) (Dallner and Sindelar, 2000); cis-prenyltransferase, which leads to the formation of dolichol and dolichyl phosphate (Krag, 1998) and heme O synthase, which results in the conversion of heme B to heme O and heme A (Brown, et al., 2002). In addition, FPP can be dephosphorylated to farnesol by one or more lipid phosphatases that have not yet been formally identified (Bansal and Vaidya, 1994), although recombinant human phosphatidic acid phosphatase 2a (PAP2a) has been shown to be capable of catalyzing this reaction in vitro (Fukunaga, et al., 2006). Farnesol can then be sequentially oxidized to farnesal, farnesoic acid and a family of dioic acids that are excreted in large quantities by animals treated with squalestatin 1 (Vaidya, et al., 1998; Bostedor, et al., 1997).
Alendronate is an NBP that inhibits FDPS with nanomolar potency (Keller and Fliesler, 1999; Bergstrom, et al., 2000). Alendronate and other drugs of its class, including risedronate and zoledronate, are used clinically for the prevention and treatment of osteoporosis, and this therapeutic efficacy is thought to be the result of FDPS inhibition, which decreases the availability of FPP and GGPP for protein prenylation reactions, which in turn leads to activation of osteoclast apoptosis (Russell, et al., 2007). Because FDPS not only catalyzes the formation of FPP, but also catalyzes the preceding metabolic reaction in which IPP and DMAPP condense to form GPP, alendronate treatment causes the accumulation of IPP and DMAPP (Keller and Fliesler, 1999; Bergstrom, et al., 2000).
In this study, we used alendronate to test whether squalestatin 1-mediated CYP2B induction in primary cultured rat hepatocytes is dependent upon the production of FPP. We report not only that squalestatin 1-mediated CYP2B induction is effectively abolished by alendronate treatment, but also that phenobarbital-mediated CYP2B induction is partially suppressed, suggesting that one or more upstream isoprenoids (e.g., IPP or DMAPP) function as endogenous suppressors of CYP2B induction.
Materials and Methods
Materials
Squalestatin 1 and pravastatin were gifts from GlaxoSmith-Kline (Research Triangle Park, NC) and Bristol-Myers Squibb Co. Stamford, CT), respectively. Alendronate, phenobarbital, mevalonate, FPP, dithiothreitol, octyl-β-D-glucopyranoside, MTT, NADPH, 7-pentoxyresorufin and resorufin sodium salt were purchased from Sigma-Aldrich (St. Louis, MO). PureCol was purchased from Inamed (Fremont, CA). Matrigel and Cell Recovery Solution were purchased from BD Biosciences (Bedford, MA). Culture medium was purchased from Invitrogen (Carlsbad, CA). Recombinant human insulin (Novolin R) was purchased from Novo Nordisk Pharmaceuticals, Inc (Princeton, NJ). Nylon hybridization filters (Gene Screen Plus) and [α-32P]dATP were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Solvents for lipid extraction and chromatography (Burdick and Jackson) were purchased from Fisher Scientific (Hanover Park, IL). Other materials were purchased from the sources indicated below.
Primary culture of rat hepatocytes
Hepatocytes were isolated from adult male Sprague-Dawley rats (250-350 g, Harlan, Indianapolis, IN) as previously described (Kocarek and Reddy, 1996). Freshly isolated hepatocytes were suspended in Williams' Medium E supplemented with 0.25 U/ml insulin, 10−7 M triamcinolone acetonide, 100u/ml penicillin and 100ug/ml streptomycin and plated into collagen (PureCol; 99.9% collagen; ∼97% type I collagen with remainder type III collagen)-coated plastic vessels. Three million viable hepatocytes were plated into 60-mm dishes (for FPP measurements and microsome preparation), 1.5 million cells were plated into the wells of 6-well plates (for RNA isolation) and 350,000 cells were plated into the wells of 12-well plates (for MTT assays). Twenty-four hr after plating, the medium was replaced with fresh medium containing 0.2 mg/ml Matrigel. The medium was thereafter replaced (without Matrigel) at 24 hr intervals. Drug treatments were initiated 48 hr post-plating, and were performed as described in the individual figure legends. Drugs were added to the culture medium as concentrated stock solutions in water.
Northern and western blot analysis
Total RNA was isolated from hepatocytes cultured in 6-well plates using TRIzol Reagent (Invitrogen), and the RNA samples constituting each treatment group (3 wells of hepatocytes per group) were pooled. Ten μg samples of RNA were resolved on denaturing agarose gels and analyzed by northern blot hybridization, using the procedures and cDNA probes to CYP2B1, CYP3A23, HMG-CoA reductase and 7S that have been described previously (Kocarek and Reddy, 1996). Because the CYP2B1 and CYP3A23 cDNA probes cross-react with multiple related transcripts, the bands detected with these probes are referred to as CYP2B and CYP3A. The CYP2B1 probe most probably hybridizes with CYP2B1 and CYP2B2 transcripts on northern blots of primary cultured male rat hepatocyte RNA, while the CYP3A23 cDNA probe most probably detects CYP3A1, CYP3A2 and CYP3A23 transcripts on these northern blots. CYP2B and 7S band intensities were measured using an Image Station 440CF with 1D Image Analysis Software, v. 3.6 (Eastman Kodak Co., Rochester, NY), and the CYP2B values were normalized to the corresponding 7S values.
For microsome isolation, hepatocytes in 60 mm dishes were washed once with cold PBS and scraped into one ml cold Cell Recovery Solution. The hepatocytes constituting each treatment group (two dishes of hepatocytes per group) were pooled and gently mixed by inversion for 15 min 4°C to remove traces of Matrigel. The hepatocytes were then pelleted, washed once with cold PBS and stored at −80°C until further processing. Cell pellets were suspended in 100 μl 50 mM Tris-HCl, 1 mM EDTA, 0.25 M sucrose, pH 7.4, and homogenized by sonication. Homogenates were centrifuged for 20 min at 20,000 × g at 4°C, and supernatants were centrifuged for 1 hr at 105,000 × g at 4°C. The microsomal pellets were suspended in 40 μl 50 mM Tris-HCl, 20% glycerol and 1 mM EDTA, pH 7.4, and protein concentrations were measured using the Quickstart Bradford Protein Assay (BioRad, Hercules, CA). SDS-PAGE was performed using 10% Tris-HCl 12-well Ready-Gels (BioRad) and 2 μg microsomal protein per well, and resolved proteins were transferred onto Immuno-Blot PVDF membranes (BioRad). Immunoreactive CYP2B proteins were detected using a polyclonal antibody to CYP2B1 (Xenotech LLC, Lenexa, KS) and the ECL Plus Western Blotting Detection system (Amersham Biosciences Inc., Piscataway, NJ).
PROD activity
Reaction mixes were assembled in 96-well fluorescence plates and contained 20 μg microsomal protein, 10 μM pentoxyresorufin, 5 mM MgCl2, 50 mM Tris-HCl, pH 7.5, and 1mM NADPH (added last to initiate the reactions) in a total volume of 200 μl. Reactions were incubated in a SpectraMax Gemini fluorescence plate reader (Molecular Devices Corporation, Sunnyvale, CA) at 37°C, and fluorescence data (excitation wavelength = 522 nm; emission wavelength = 586 nm) were acquired every 45 sec using SoftMax Pro software (Molecular Devices). Slopes were fit to the initial, linear portions of the fluorescence–time plots, and data were expressed as pmol resorufin formed/min/mg microsomal protein (based on comparison to a standard curve generated by measuring the fluorescence of wells containing 0-5 pmol resorufin in buffer). PROD activities were undetectable in samples incubated without NADPH.
MTT activity
Hepatocytes cultured in 24-well plates were incubated with 0.3 ml 2.4 mM MTT in PBS for 30 min at 37°C, washed once with PBS, and extracted with 0.3 ml isopropanol with continuous shaking at room temperature for 30 min. Plates were centrifuged at 3000 rpm for 10 min, and 40 μl aliquots of the supernatants were added to 160 μl isopropanol in the wells of a 96-well plate. Absorbance was measured at 560 nm using a SpectraMax Plus Absorbance Microplate Reader and SoftMax Pro software (Molecular Devices).
Measurement of FPP levels
Cellular levels of FPP were measured according to the method described by Tong et al. (Tong, et al., 2005). Following treatment, hepatocytes were recovered from 60 mm dishes using Cell Recovery Solution, as described above. The hepatocytes constituting each treatment group (three dishes of hepatocytes per group) were pooled. Cellular protein contents were measured using the bicinchoninic acid assay (Smith, et al., 1985). Cell pellets were extracted twice at 70°C with butanol-75 mM ammonium hydroxide-ethanol (1:1.25:2.75 v/v/v). Pooled supernatants were dried under nitrogen at 50°C and residues were dissolved in 50 mM Tris-HCl, pH 7.5, containing 5 mM dithiothreitol, 5 mM MgCl2, 10 μM ZnCl2, and 1% octyl-β-D-glucopyranoside. Each farnesylation reaction contained extract equivalent to 200 μg cellular protein, 500 pmol N-dansylated carboxyl terminus of H-Ras peptide (DansylGCVLS, Sigma-Aldrich) and 136 units (250 ng) recombinant rat protein farnesyl transferase (Calbiochem, La Jolla, CA) in a total volume of 50 μl. Additional reactions containing known quantities of FPP (7-42 pmol) were analyzed in parallel to the samples, to permit construction of standard curves. Reactions were performed at 38°C for 120 min and terminated by addition of 50 μl acetonitrile and 5 μl of 1.2 N hydrochloric acid. Denatured proteins were removed by centrifugation and 20 μl aliquots of supernatants were analyzed by high performance liquid chromatography, using a Waters system (1525 Binary HPLC pump, 2487 Dual λ Absorbance Detector, busSAT/IN Module and Breeze software) and a Shimadzu RF-10AXL fluorescence detector. Separations were performed using a Symmetry C18 5.0μm, 4.6mm × 150mm column protected by a guard column (Waters) and a binary mobile phase consisting of solvent A: 25 mM ammonium acetate in 40% acetonitrile and solvent B: 20 mM ammonium acetate in 90% acetonitrile. Chromatography was performed at room temperature with a mobile phase flow rate of 1 ml/min, and was initiated upon sample injection with 38% B for 1 min, followed by linear transition over 7 min to 100% B, followed by 100% B for 8 min, followed by return to initial conditions over 3 min, followed by re-equilibration. The free and farnesylated peptides were detected using an excitation wavelength of 335 nm and an emission wavelength of 528 nm. The retention time for the farnesylated peptide varied from 5.5 to 6.4 minutes, depending on the ambient temperature. Peak areas were determined, and data were calculated as pmol FPP per mg cellular protein.
Statistical analysis
Numerical data (FPP levels, MTT activities and normalized northern blot data) from replicate experiments (i.e., from multiple independent hepatocyte preparations) were analyzed using one-way analysis of variance followed by the Newman-Keuls Multiple Comparison Test, with p<0.05 set as the level of significance. For this purpose, one treatment group was defined as the reference group, and the other treatment group data were expressed as percentages of the reference group value within each experiment. Therefore, the reference groups (defined as 100% in each experiment; therefore no inter-experiment variability) were excluded from the analyses, as were individual groups containing fewer than three samples. IC50 values were determined using the log(inhibitor) vs. response-variable slope function of Prism 5.0 (GraphPad Software, Inc., La Jolla, CA).
Results
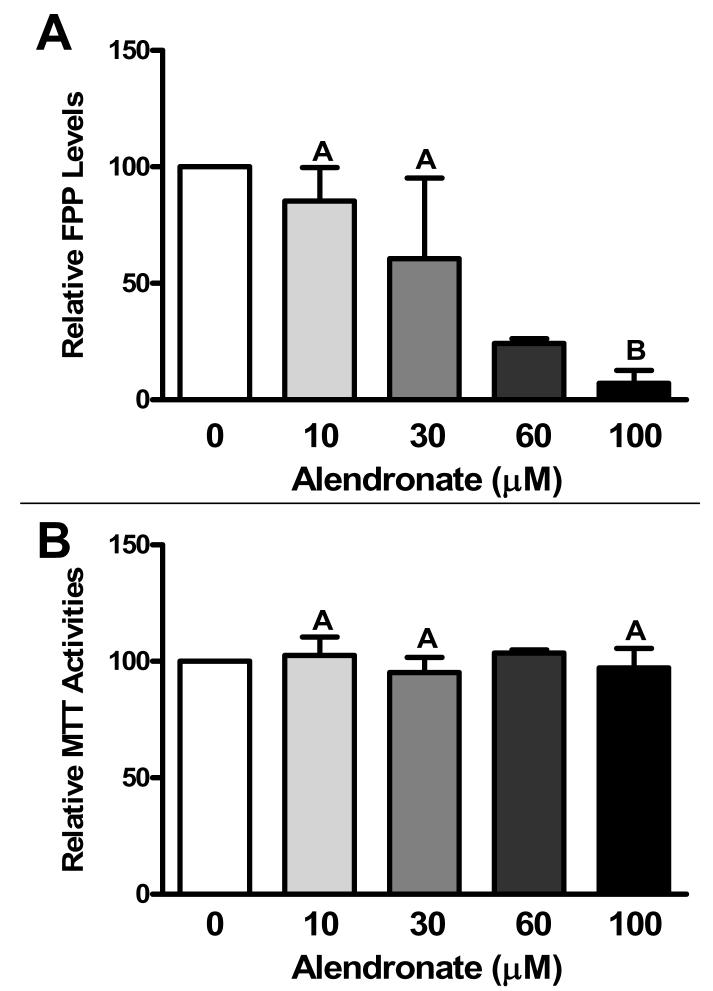
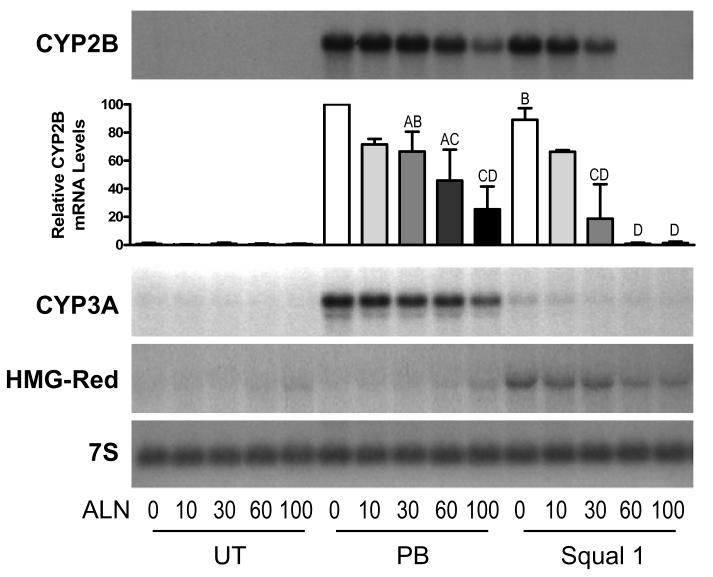
Initial experiments were performed to determine the ability of alendronate to produce effective inhibition of FDPS activity in primary cultured rat hepatocytes, using cellular FPP content as the criterion. Because untreated primary rat hepatocyte cultures contained very low levels of FPP, and treatment with 0.1 μM squalestatin 1 caused substantial FPP accumulation (10- to 20-fold, data not shown), the concentration-dependent ability of alendronate to reduce squalestatin 1-mediated FPP accumulation was evaluated. Treatment of rat hepatocyte cultures with alendronate concentrations lower than 10 μM had no effect on squalestatin 1-mediated FPP accumulation (data not shown), but treatment with alendronate concentrations of 10 to 100 μM produced a concentration-dependent reduction of FPP levels (IC50 ∼40 μM), with average decreases of ∼75% and >90% occurring at alendronate concentrations of 60 and 100 μM, respectively (Fig. 2A). These effective alendronate concentrations were not toxic to the hepatocytes, as judged by the lack of effect on MTT activity (Fig. 2B). Alendronate was next evaluated for its ability to modify CYP2B, CYP3A and HMG-CoA reductase expression, either when used alone or in combination with 0.1 μM squalestatin 1 or 100 μM phenobarbital (Fig. 3). Treatment with alendronate alone had no effect on CYP2B or CYP3A mRNA content (Fig. 3). However, co-treatment with alendronate effectively inhibited squalestatin 1-mediated CYP2B induction (IC50∼30 μM), with complete suppression occurring at alendronate concentrations of 60 and 100 μM (Fig. 3). In addition, alendronate treatment produced a concentration-dependent suppression of phenobarbital-mediated CYP2B induction, which, although, less pronounced than the absolute blockade that occurred for squalestatin 1-mediated induction, was nevertheless significant at an alendronate concentration of 100 μM (Fig. 3). Although phenobarbital is commonly regarded as an inducer of both CYP2B and CYP3A, this drug induces CYP2B and CYP3A with different concentration-dependencies (Kocarek, et al., 1990). Thus, while maximal CYP2B induction is achieved at 100 μM phenobarbital, this concentration produces only a slight elevation of CYP3A expression. Alendronate co-treatment suppressed the phenobarbital-mediated CYP3A induction that did occur in parallel with the above-described suppression of CYP2B induction (Fig. 3). As an additional observation, squalestatin 1 treatment produced the expected up-regulation of HMG-CoA reductase expression that signifies effective blockade of cholesterol biosynthesis (Fig. 3). By contrast, treatment with alendronate produced only very slight up-regulation of HMG-CoA reductase expression, even at a concentration (100 μM) that produced near complete inhibition of farnesyl diphosphate synthase (Figs 2 and 3). Moreover, alendronate co-treatment produced a concentration-dependent suppression of the squalestatin 1-mediated HMG-CoA reductase up-regulation that was in close correspondence to alendronate's suppressive effects on phenobarbital-inducible CYP2B and CYP3A expression (Fig. 3).
Fig. 2.
Concentration-dependent effects of alendronate treatment on FPP levels and MTT activities in primary cultured rat hepatocytes. At 48 hr after plating, primary cultured rat hepatocytes were incubated for 48 hr in medium containing 0.1 μM squalestatin 1 alone (0) or in combination with 10, 30, 60 or 100 μM alendronate, after which they were harvested for measurement of FPP levels (A) or MTT activities (B). Data represent the mean values obtained in four independent hepatocyte preparations ± sd (except for the 60 μM values, which represent the mean ± range of two hepatocyte preparations) relative to control (0 μM alendronate). Groups not sharing a letter are significantly different from each other, p<0.05.
Fig. 3.
Concentration-dependent effects of alendronate treatment on squalestatin 1- and phenobarbital-inducible CYP2B, CYP3A and HMG-CoA reductase mRNA levels in primary cultured rat hepatocytes. At 48 hr after plating, primary cultured rat hepatocytes were incubated for 48 hr in medium alone (UT) or containing 100 μM phenobarbital (PB) or 0.1 μM squalestatin 1 (Squal 1), alone (0) or in combination with 10, 30, 60 or 100 μM alendronate (ALN). After treatment, hepatocytes were harvested for measurement of CYP2B, CYP3A or HMG-CoA reductase (HMG-Red) mRNA levels by northern blot hybridization. Individual panels show autoradiographs from a representative experiment; also shown is an autoradiograph from the CYP2B blot that had been hybridized with 7S cDNA probe, to demonstrate consistency of RNA loading and transfer among samples. Also shown is a graphical representation of the densitometrically quantified CYP2B mRNA content data from three independent hepatocyte preparations (except for the 10 μM ALN values, which are from two preparations). Each bar represents the mean ± sd CYP2B mRNA content as a percentage of the group treated with PB alone (PB, 0), except for the PB, 0 group itself (defined as 100% in each experiment) and the 10 μM ALN groups (presented as mean ± range). Groups not sharing a letter are significantly different from each other, p<0.05.
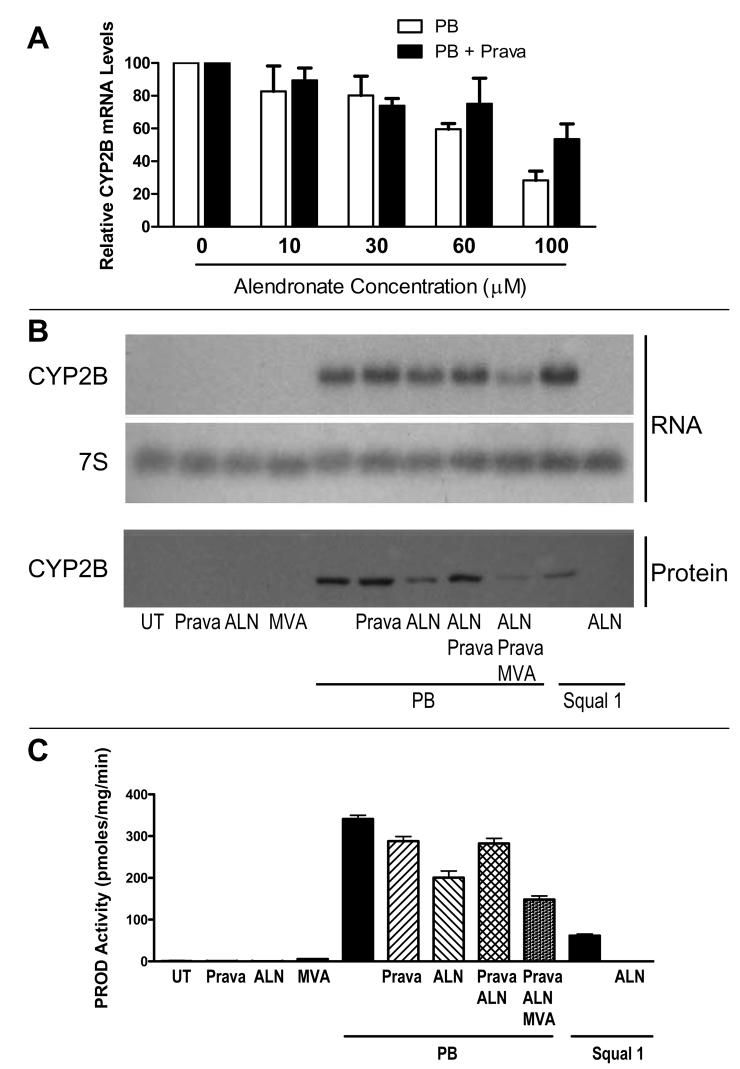
The partial suppression of phenobarbital-mediated CYP2B induction that was produced by alendronate co-treatment prompted us to consider whether the effect was produced by alendronate itself, or rather was a consequence of FDPS inhibition, as NBP treatments have been reported to cause accumulation of upstream isoprenoids, including isopentenyl pyrophosphate and dimethylallyl pyrophosphate (Keller and Fliesler, 1999; Bergstrom, et al., 2000). To discriminate between these possibilities, we performed treatments with the HMG-CoA reductase inhibitor, pravastatin, to shut off the cholesterol biosynthetic pathway at the level of mevalonate and thereby prevent the ability of alendronate to cause accumulation of any isoprenoids. Pravastatin was specifically selected for use, as this drug produces effective upstream blockade of the cholesterol biosynthetic pathway but does not itself produce any effects on CYP2B expression (Kocarek and Reddy, 1996; Kocarek, et al., 2002). The concentration-dependent ability of alendronate to suppress phenobarbital-mediated CYP2B induction was evaluated in the absence and presence of pravastatin (Fig. 4A). As previously seen, alendronate treatment produced concentration-dependent suppression of phenobarbital-mediated CYP2B induction. This suppression was attenuated when the hepatocytes were co-treated with pravastatin. In particular, the suppressive effect that was produced by 60 μM alendronate was largely prevented by pravastatin co-treatment, while the greater suppression that was produced by 100 μM alendronate treatment was partially reversed by pravastatin (Fig. 4A). Next, the effects of pravastatin-mediated HMG-CoA reductase blockade, combined with rescue by mevalonate treatment, were evaluated on alendronate (60 μM)-mediated suppression of phenobarbital-inducible CYP2B mRNA and immunoreactive protein levels (Fig. 4B) and CYP2B-mediated catalytic activity (Fig. 4C). Alendronate-mediated suppression of phenobarbital-inducible CYP2B expression was evident at all levels of measurement, as was pravastatin-mediated reversal of the suppression. Co-treatment of pravastatin-treated hepatocyte cultures with mevalonate restored alendronate's ability to cause suppression of phenobarbital-mediated CYP2B induction (Fig. 4B and C). Squalestatin 1-mediated induction of CYP2B mRNA, protein and enzymatic activity was abolished by treatment with 60 μM alendronate (Fig. 4B and C).
Fig. 4.
Effect of pravastatin co-treatment on alendronate-mediated suppression of phenobarbital-inducible CYP2B expression. A. At 48 hr after plating, primary cultured rat hepatocytes were incubated for 48 hr in medium containing 100 μM phenobarbital (PB) or PB and 30 μM pravastatin (Prava), alone (0) or in combination with 10, 30, 60 or 100 μM alendronate. After treatment, hepatocytes were harvested for measurement of CYP2B mRNA levels by northern blot hybridization. Each bar represents the mean ± range (data are from two independent hepatocyte preparations) CYP2B mRNA content as a percentage of either the corresponding group treated with PB alone (PB, 0) or with PB+Prava alone (PB+Prava, 0). B and C. Primary cultured rat hepatocytes were incubated for 48 hr in medium alone (UT) or containing 100 μM PB or 0.1 μM squalestatin 1 (Squal 1), alone or in combination with one or more of the following: 30 μM Prava, 60 μM alendronate (ALN) or 10 mM mevalonate (MVA). After treatment, hepatocytes were harvested for the measurement of CYP2B mRNA and immunoreactive protein contents (B) and PROD activities (C). PROD activities represent the mean ± sd of triplicate measurements. Comparable results were obtained in a second hepatocyte preparation.
Discussion
Alendronate treatment alone had no effect on CYP2B or CYP3A mRNA expression in primary cultured rat hepatocytes, but completely suppressed squalestatin 1-inducible CYP2B expression at the same concentrations that inhibited FDPS. Although, given alendronate's reported nanomolar potency for FPDS inhibition in vitro (Keller and Fliesler, 1999; Bergstrom, et al., 2000), the alendronate concentrations (10-100 μM) that caused effective FPDS inhibition in cultured rat hepatocytes may seem high, these concentrations are comparable to those found to produce FPDS inhibition in cultured murine osteoclasts (Bergstrom, et al., 2000). The complete blockade of squalestatin 1-mediated CYP2B induction that was produced by alendronate treatment is consistent with a requirement for ongoing FPP synthesis in the induction process, although a caveat is that FDPS inhibition prevents the production of both GPP and FPP. Nevertheless, these findings clearly demonstrate that squalestatin 1 is incapable of causing CYP2B induction unless isoprenoid biosynthesis is allowed to proceed at least to the level of GPP.
Phenobarbital was included in this study as a positive control CAR activator and CYP2B inducer, whose effect on CYP2B expression is unaffected by co-treatment with the upstream cholesterol biosynthesis inhibitor, pravastatin (Kocarek and Mercer-Haines, 2002). Our expectation was that phenobarbital-mediated CYP2B induction, in contrast to squalestatin 1-mediated induction, would be unaltered by alendronate co-treatment. However, alendronate treatment was found to produce a concentration-dependent suppression of phenobarbital-mediated CYP2B induction that, while less pronounced than the complete blockade of squalestatin 1-mediated induction that occurred, was nevertheless substantial. This finding prompted us to investigate whether the suppressive effect of alendronate on phenobarbital-mediated CYP2B induction could be attributed to a direct action of alendronate itself, or rather whether the suppression was transduced as a consequence of FPDS blockade and accumulation of an upstream metabolite. Co-treatment of hepatocyte cultures with pravastatin, to block mevalonate, and therefore all isoprenoid, biosynthesis, attenuated the ability of alendronate to suppress phenobarbital-inducible CYP2B expression, and this attenuation was overridden by bypassing the pravastatin-mediated blockade through the addition of exogenous mevalonate. Pravastatin treatment effectively reversed the suppressive effect on phenobarbital-mediated CYP2B expression that was produced by 60 μM alendronate, but only partially reversed the larger suppressive effect that was produced by 100 μM alendronate. These findings indicate that alendronate's suppressive effects on phenobarbital-mediated CYP2B induction can be attributed both to FDPS inhibition with upstream metabolite accumulation (i.e., since some of the effect can be reversed by upstream blockade) and to direct drug action on the induction process (i.e., since some of the effect is not inhibited by pravastatin treatment).
The nature of the alendronate-generated endogenous bioactive suppressor of phenobarbital-mediated CYP2B induction can be surmised by the knowledge that alendronate treatment has been shown to cause accumulation of the upstream isoprenoids, IPP and DMAPP (Keller and Fliesler, 1999; Bergstrom, et al., 2000). To date, IPP and/or DMAPP have been implicated as biologically active molecules in at least two ways. First, IPP and DMAPP have been identified as probable mediators of adverse inflammatory effects that are produced by NBP treatments. NBPs stimulate VγVδ2 T-cells to produce inflammatory cytokines in a manner that can be prevented by statin treatment and mimicked by IPP or DMAPP (Tanaka, et al., 1995; Burk, et al., 1995; Kunzmann, et al., 2000; Thompson and Rogers, 2004; Hewitt, et al., 2005). It has been suggested that this action may underlie the rare occurrence of musculoskeletal adverse effects that occur in patients taking NBPs (Bock, et al., 2007). IPP has also been identified as a tumor cell antigen that causes activation of VγVδ2 T-cells (Gober, et al., 2003), which has implications for the mechanisms governing immunosurveillance in response to the metabolic derangements occurring in cancer cells. NBPs also cause necrotic and inflammatory effects in rats and mice, although these species lack VγVδ2 T-cells, and IPP also appears to underlie at least some of these effects (Deng, et al., 2007). Such inflammatory effects could conceivably confound investigation of the ability of NBP treatment to modify hepatic P450 regulation in vivo.
Second, IPP is able to combine with adenosine monophosphate, in a reaction catalyzed by aminoacyl-tRNA synthases, to form a novel ATP analog, termed triphosphoric acid 1-adenosin-5'-yl ester 3-(3-methylbut-3-enyl) ester, or ApppI (Monkkonen, et al., 2006). ApppI has been identified in extracts of cultured osteoclasts, macrophages and glioma cells treated with an NBP (Monkkonen, et al., 2006), as well as in peritoneal macrophages isolated from zoledronate-treated mice (Monkkonen, et al., 2007). ApppI has been shown to induce apoptosis in osteoclast cells as a result of inhibition of the mitochondrial adenine nucleotide translocase (Monkkonen, et al., 2006). Therefore, NBP-mediated osteoclast apoptosis is likely the result of two mechanisms acting in combination: i.e., reduction of FPP and GGPP levels, which reduces the prenylation of critical proteins, and formation of ApppI, which causes disruption of adenosine nucleotide transport and mitochondrial function.
Whether or how either of the two above-described actions may contribute to the alendronate-mediated suppressive effects on CYP2B induction that we report in this study is a topic for further investigation. Since current evidence suggests that CAR can be activated by processes that cause activation of the energy-sensing AMP-activated protein kinase (Rencurel, et al., 2005; Rencurel, et al., 2006; Shindo, et al., 2007; Blattler, et al., 2007), it seems possible that ApppI formation, followed by inhibition of adenine nucleotide translocase and alteration of hepatocellular ATP/AMP balance may contribute to the mechanism whereby alendronate treatment causes suppression of phenobarbital-inducible CYP2B expression. An additional possibility is that alendronate and/or one or more of the upstream isoprenoids are able to interact with CAR as inverse agonists. However, our initial experiments, using an in vitro human CAR interaction assay, have not supported the operation of such a suppressive mechanism.
It is clear that endogenous isoprenoids are capable of producing diverse effects on hepatic P450 expression. Achieving an understanding of the underlying mechanisms will both enhance our knowledge about the biological capacity of this complex series of endogenous molecules and improve our ability to predict the impact of isoprenoid-modifying treatments on xenobiotic metabolism.
Acknowledgments
This work was conducted with support from National Institutes of Health Grant HL50710 and services provided by the Cell Culture and Gene Transfer Technologies Facility Core and the Imaging and Cytometry Facility Core of EHS Center Grant ES06639.
1Abbreviations used are
- CAR
constitutive androstane receptor (or NR1I3)
- DMAPP
dimethylallyl pyrophosphate
- FDPS
farnesyl diphosphate synthase (or farnesyl pyrophosphate synthase)
- FPP
farnesyl pyrophosphate (or farnesyl diphosphate)
- GGPP
geranylgeranyl pyrophosphate
- GPP
geranyl pyrophosphate
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- IPP
isopentenyl pyrophosphate
- MTT
3-(4,5-di methylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- NBP
nitrogen-containing bisphosphonate (or aminobisphosphonate)
- PBS
phosphate-buffered saline
- PROD
pentoxyresorufin O-depentylase
References
- Bansal VS, Vaidya S. Characterization of two distinct allyl pyrophosphatase activities from rat liver microsomes. Arch Biochem Biophys. 1994;315:393–399. doi: 10.1006/abbi.1994.1516. [DOI] [PubMed] [Google Scholar]
- Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys. 2000;373:231–241. doi: 10.1006/abbi.1999.1502. [DOI] [PubMed] [Google Scholar]
- Blattler SM, Rencurel F, Kaufmann MR, Meyer UA. In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104:1045–1050. doi: 10.1073/pnas.0610216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Boerst H, Thomasius FE, Degner C, Stephan-Oelkers M, Valentine SM, Felsenberg D. Common musculoskeletal adverse effects of oral treatment with once weekly alendronate and risedronate in patients with osteoporosis and ways for their prevention. J Musculoskelet Neuronal Interact. 2007;7:144–148. [PubMed] [Google Scholar]
- Bostedor RG, Karkas JD, Arison BH, Bansal VS, Vaidya S, Germershausen JI, Kurtz MM, Bergstrom JD. Farnesol-derived dicarboxylic acids in the urine of animals treated with zaragozic acid A or with farnesol. J Biol Chem. 1997;272:9197–9203. doi: 10.1074/jbc.272.14.9197. [DOI] [PubMed] [Google Scholar]
- Brown KR, Allan BM, Do P, Hegg EL. Identification of novel hemes generated by heme A synthase: evidence for two successive monooxygenase reactions. Biochemistry. 2002;41:10906–10913. doi: 10.1021/bi0203536. [DOI] [PubMed] [Google Scholar]
- Burk MR, Mori L, De Libero G. Human Vγ9δ2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052–2058. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- Dallner G, Sindelar PJ. Regulation of ubiquinone metabolism. Free Radic Biol Med. 2000;29:285–294. doi: 10.1016/s0891-5849(00)00307-5. [DOI] [PubMed] [Google Scholar]
- Deng X, Yu Z, Funayama H, Yamaguchi K, Sasano T, Sugawara S, Endo Y. Histidine decarboxylase-stimulating and inflammatory effects of alendronate in mice: Involvement of mevalonate pathway, TNFα, macrophages, and T-cells. Int Immunopharmacol. 2007;7:152–161. doi: 10.1016/j.intimp.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Arita M, Takahashi M, Morris AJ, Pfeffer M, Levy BD. Identification and functional characterization of a presqualene diphosphate phosphatase. J Biol Chem. 2006;281:9490–9497. doi: 10.1074/jbc.M512970200. [DOI] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK. The bisphosphonate acute phase response: Rapid and copious production of proinflammatory cytokines by peripheral blood γδ T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol. 2005;139:101–111. doi: 10.1111/j.1365-2249.2005.02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh KL, Dunford JE, Bunkoczi G, Russell RG, Oppermann U. The crystal structure of human geranylgeranyl pyrophosphate synthase reveals a novel hexameric arrangement and inhibitory product binding. J Biol Chem. 2006;281:22004–22012. doi: 10.1074/jbc.M602603200. [DOI] [PubMed] [Google Scholar]
- Keller RK, Fliesler SJ. Mechanism of aminobisphosphonate action: characterization of alendronate inhibition of the isoprenoid pathway. Biochem Biophys Res Commun. 1999;266:560–563. doi: 10.1006/bbrc.1999.1849. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Dahn MS, Cai H, Strom SC, Mercer-Haines NA. Regulation of CYP2B6 and CYP3A expression by hydroxymethylglutaryl coenzyme A inhibitors in primary cultured human hepatocytes. Drug Metab Dispos. 2002;30:1400–1405. doi: 10.1124/dmd.30.12.1400. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Kraniak JM, Reddy AB. Regulation of rat hepatic cytochrome P450 expression by sterol biosynthesis inhibition: inhibitors of squalene synthase are potent inducers of CYP2B expression in primary cultured rat hepatocytes and rat liver. Mol Pharmacol. 1998;54:474–484. doi: 10.1124/mol.54.3.474. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Mercer-Haines NA. Squalestatin 1-inducible expression of rat CYP2B: evidence that an endogenous isoprenoid is an activator of the constitutive androstane receptor. Mol Pharmacol. 2002;62:1177–1186. doi: 10.1124/mol.62.5.1177. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Reddy AB. Regulation of cytochrome P450 expression by inhibitors of hydroxymethylglutaryl-coenzyme A reductase in primary cultured rat hepatocytes and in rat liver. Drug Metab Dispos. 1996;24:1197–1204. [PubMed] [Google Scholar]
- Kocarek TA, Schuetz EG, Guzelian PS. Differentiated induction of cytochrome P450b/e and P450p mRNAs by dose of phenobarbital in primary cultures of adult rat hepatocytes. Mol Pharmacol. 1990;38:440–444. [PubMed] [Google Scholar]
- Kodama S, Negishi M. Phenobarbital confers its diverse effects by activating the orphan nuclear receptor car. Drug Metab Rev. 2006;38:75–87. doi: 10.1080/03602530600569851. [DOI] [PubMed] [Google Scholar]
- Krag SS. The importance of being dolichol. Biochem Biophys Res Commun. 1998;243:1–5. doi: 10.1006/bbrc.1997.7828. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- Monkkonen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsalainen J, Monkkonen J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Brit J Pharmacol. 2006;147:437–445. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkkonen H, Ottewell PD, Kuokkanen J, Monkkonen J, Auriola S, Holen I. Zoledronic acid-induced IPP/ApppI production in vivo. Life Sci. 2007;81:1066–1070. doi: 10.1016/j.lfs.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, da S,X, Rutter GA, Viollet B, Meyer UA. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- Shindo S, Numazawa S, Yoshida T. A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem J. 2007;401:735–741. doi: 10.1042/BJ20061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MD, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced γ,δ-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- Tong H, Holstein SA, Hohl RJ. Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Anal Biochem. 2005;336:51–59. doi: 10.1016/j.ab.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Vaidya S, Bostedor R, Kurtz MM, Bergstrom JD, Bansal VS. Massive production of farnesol-derived dicarboxylic acids in mice treated with the squalene synthase inhibitor zaragozic acid A. Arch Biochem Biophys. 1998;355:84–92. doi: 10.1006/abbi.1998.0704. [DOI] [PubMed] [Google Scholar]