Summary
The two main spectral components of the advertisement calls of two species of North American gray treefrogs (Hyla chrysoscelis and H. versicolor) overlap broadly in frequency, and each matches the sensitivity of one of the two different auditory inner ear organs. The calls of the two species differ in the shape and repetition-rate (pulse rate) of sound pulses within trills. A standard synthetic call with one of these spectral peaks and the pulse rate typical of conspecific calls was tested against synthetic alternatives that had the same spectral peak but a different pulse rate. The results were generalized over a wide range of playback levels. Selectivity based on differences in pulse rate depended on which spectral peak was used in some tests, and greater pulse-rate selectivity was usually observed when the low-frequency rather than the high-frequency peak was used. This effect was more pronounced and occurred over a wider range of playback levels in H. versicolor than in H. chrysoscelis when the pulse rate of the alternative was higher than that of the standard call. In tests using the high-frequency peak at high playback levels, however, females of H. versicolor showed greater selectivity for the standard call than did H. chrysoscelis when the pulse rate of the alternative was modestly lower than that of the standard call. This last result may reflect the different ways in which females of the two species assess trains of pulses.
Keywords: phonotactic selectivity, fine-scale temporal properties, pulse rate, carrier-frequency, interactive effects, tree frogs (Hylidae)
Introduction
Intraspecific communication involves the transmission of signals that potentially convey information about the sender's species, gender, reproductive status, and fitness – both physical and genetic. Acoustic signals typically encode such information by modulation (amplitude [AM], frequency [FM] or both) of one or more carrier components or, frequency bands that contain all or a substantial proportion of the total acoustic energy (Bradbury and Vehrencamp, 1998). The spectral structure (number of spectral peaks and their frequency and relative amplitudes) may also contribute to signal recognition, and carrier frequency may provide reliable information about the size of the signaler (Gerhardt and Huber, 2002). In most experimental studies of intraspecific acoustic communication, the spectral properties of test stimuli are held constant while the temporal properties are varied or vice-versa. This design reflects two implicit hypotheses: (1) that any frequency component or band to which the auditory system is reasonably sensitive may serve as a carrier of information encoded in the temporal properties of the signal; and (2) that receivers process and make decisions based on fine-scale temporal properties independently of differences in spectral properties and vice-versa. In this study, I explicitly test the null hypothesis that receiver selectivity based on pulse rate, which is almost certainly processed in the time domain by the experimental subjects (Gerhardt, 1978; see Discussion) is independent of that based on carrier frequency. The experiments also address the question of whether selectivity based on differences in pulse rate decreases when the number of spectral peaks typical of conspecific signals is reduced.
Frogs and toads have served as important model systems for the study of both evolution and auditory mechanisms because of their strong reliance on acoustic communication (reviews: Gerhardt and Huber, 2002; Narins et al., 2006). The anuran auditory system is specialized at the peripheral level by virtue of having two inner ear organs with different frequency sensitivities and physiological properties. In species in which the advertisement call has a bimodal spectrum, the frequency of the high-frequency spectral peak usually matches the tuning of the basilar papilla, whereas the frequency of the low-frequency peak usually matches the tuning of relatively high-frequency-tuned neurons in the amphibian papilla (reviews: Capranica and Moffat, 1983; Gerhardt and Schwartz, 2001). Furthermore, these organs have different physiological properties, including a difference in their potential to encode rapid temporal changes by phase-locking (review: Simmons et al., 2006).
In this study, I assessed the preferences of two cryptic species of North American gray treefrogs (Anura: Hylidae). Cope's gray treefrog (H. chrysoscelis Cope) is a diploid species in which males produce pulsed advertisement calls with a relatively rapid pulse rate (about 35 to 70 pulses/s uncorrected for temperature). The second species (H. versicolor LeConte) is a tetraploid species that has had three or more independent origins from hybridization events involving H. chrysoscelis and other extinct diploid species (Ptacek et al., 1994; Holloway et al., 2006). The pulsed advertisement calls of H. versicolor have a slower pulse rate (about 10 to 35 pulses/s uncorrected for temperature) than the calls of H. chrysoscelis.
Whereas females of H. chrysoscelis base their choices on pulse rate or, equivalently, on the pulse period, which is time between the onsets of two successive pulses in sequences of pulses; females of H. versicolor, assess fine-scale temporal properties in terms of pulse duration and the silent interval between pulses (Schul and Bush, 2002). I emphasize that in the calls of both species (Gerhardt and Doherty, 1988) and in the synthetic signals I used in this study, pulse duration and the interpulse interval vary in a dependent fashion with pulse rate so that the durations of these properties are greater in a signal with a low pulse rate than in a signal with a high pulse rate (Fig. 1).
Figure 1.
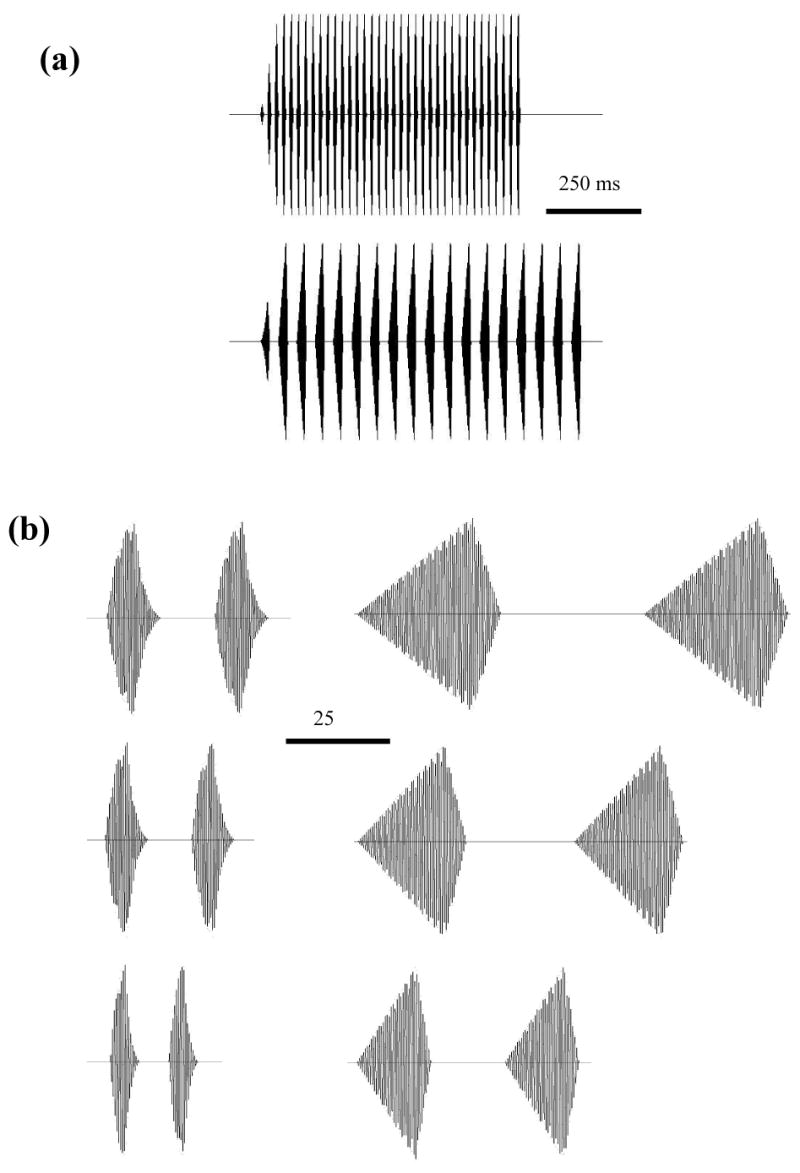
(a) Oscillograms showing the gross temporal structure of standard synthetic advertisement calls of H. chrysoscelis (top) and H. versicolor (bottom); (b) Oscillograms with an expanded time base showing two pulses from six synthetic calls. Left column: H. chrysoscelis with the 2.4-kHz carrier frequency; pulse rates were (from top to bottom): 40 pulses/s, 50 pulses/s (the standard rate), and 75 pulses/s; Right column: H. versicolor with the 2.2-kHz carrier frequency; pulse rates were (from top to bottom): 15 pulses/s, 20 pulses/s (the standard rate), and 30 pulses/s
The carrier frequencies in the calls of the two species are similar (a low-frequency carrier typically ranging from 1.0-1.4 kHz has a relative amplitude that is typically about 10 dB less than that of a harmonically related high-frequency carrier ranging from about 2.0-2.8 kHz (Gerhardt, 2005a). Females of both species preferred signals with bimodal spectra to signals having just one of these spectral peaks (Gerhardt, 2005a) but surprisingly chose single-component calls of 1.1 or 1.2 kHz rather than alternatives of 2.2 or 2.4 kHz, respectively at low playback levels (65-75 dB). Females either did not show a preference (H. chrysoscelis from most populations) or preferred the high-frequency alternatives (H. versicolor; H. chrysoscelis from Minnesota [Bee, unpubl. data]) at playback levels of 85-90 dB SPL (Gerhardt et al., 2007). These intensity-dependent changes in preference serve to emphasize that the auditory system often shows non-linear characteristics and that female selectivity should be assessed over a wide range of playback level.
Although differences in the tuning of the auditory organs suggests a two-channel system, the situation is more complicated for two reasons: (1) different kinds of receptors within the amphibian papilla have different tuning properties and respond differently to two-tone stimulation; and (2) inputs from the two inner organs already show some degree of convergence in the very first auditory nucleus in the ascending pathway. Auditory neurons in the ascending pathway show a bewildering diversity of response properties to the spectral, and especially fine-scale temporal properties of acoustic signals (e.g., Walkowiak, 1984; reviewed by Rose and Gooler, 2006). Thus, quantitative behavioral experiments, which explore the multi-variate acoustic space of signals that best elicit responses from the whole animal, are an important complementary step to understanding how auditory systems recognize biologically significant signals (see Schmidt et al. 2007 for a recent study of grasshoppers with the same perspective).
I will show that phonotactic selectivity based on differences in fine-scale temporal properties may be affected in significant ways by carrier frequency. Such effects were stronger in H. versicolor than in H. chrysoscelis. There were also significant interactions between preferences based on carrier frequency, the direction of the pulse-rate difference relative to that of the standard call, and playback level in H. versicolor but not H. chrysoscelis. Temporal selectivity was generally greater when the temporal information was carried by the low-frequency band than when it was carried by the high-frequency band. I propose that exceptions in H. versicolor may reflect differences in the way that females of this species assess fine-scale temporal patterns.
Materials and Methods
Acoustic stimuli
The physical properties of the advertisement calls of H. versicolor and H. chrysoscelis have already been well described (Gerhardt et al., 1994; Gerhardt, 2005a,b). Synthetic calls were made with custom-designed software (written by J. J. Schwartz) that created 8-bit or 16-bit digital files with an output sampling rate of at least 20 kHz. The temporal properties of standard synthetic calls were typical of calls produced by conspecific males calling at about 20° C, which was also the temperature (± 2° C) at which females were tested. The bandwidths of spectral peaks were narrower than in natural calls because the synthetic calls did not incorporate the within-pulse frequency modulation typical of these species (Figure 1). In tests of H. versicolor and H. chrysoscelis these standard calls were as attractive as synthetic calls with frequency-modulated pulses, which, in turn, were as attractive as typical pre-recorded natural calls (Gerhardt, 1978, 2005b).
For the experiments described in this study, the two standard calls used to test H. chrysoscelis had a pulse rate of 50 pulses/s and a 50% pulse-duty cycle. One standard call had a single carrier frequency of 1.2 kHz, and the other, a single carrier frequency of 2.4 kHz. Alternatives had one or the other of these carrier frequencies and pulse rates of 40, 45, 65 or 75 pulses/s; pulse duty cycle was always 50% (see examples in Figure 1). The two standard calls used to test H. versicolor had a pulse rate of 20 pulses/s and a 50% duty cycle. One standard call had a single carrier frequency of 1.1 kHz, and the other, a single carrier frequency of 2.2 kHz. Alternatives had one or the other of these carrier frequencies and pulse rates of 10, 15, 25, 30, 35 or 40 pulses/s; pulse duty cycle was always 50% (examples in Fig. 1). In all tests, both the standard and the alternative call had the same carrier frequency and differed in pulse rate. Synthetic calls used to test H. chrysoscelis had 36 pulses, and calls used to test H. versicolor had 18 pulses. Call period was 4 s in stimuli presented to both species. The timing relationship of alternatives in any given test was fixed so that there were equal periods of silence between the end of one stimulus and the beginning of the alternative stimulus.
Females of H. versicolor and H. chrysoscelis were tested in the semi-anechoic chamber described in Gerhardt (1994). Sounds were amplified by Nagra DSM amplifiers and played back from two Analog-Digital-Systems 200 speakers, which were separated by 2 m. Digital files were output from an IBM-compatible personal computer using software specific to digital-to-analog interfaces (Siliconsoft DacEditor 12AB, 16-bit). Signal amplitudes were adjusted and equalized at the female release point midway between the speakers using a General Radio 1990 or Larson-Davis 720 sound level meter. Most initial tests were conducted at a playback level of 85 dB SPL in order to compare the results with those obtained at the same level using stimuli with bimodal spectra (Gerhardt, 2005a, b). I also conducted tests at playback levels of 90, 75, and 65 dB SPL with both species. Females of H. versicolor were less likely to respond at all at 65 dB SPL than were females of H. chrysoscelis, so some tests were conducted at 69 dB SPL. Tests with sufficient data at both levels indicated that there was no significant difference in choices at these two intensities, so the results at these two levels were combined.
Experimental procedure
Female gray treefrogs were collected in amplexus at the localities specified previously in Missouri (USA) (Gerhardt et al. 2007) and refrigerated (about 4°C) to delay oviposition. Before testing (usually within 3 days of capture), each female was acclimated to the target test temperature (20°C) for at least 30 min. Each female was placed in a circular, acoustically transparent (hardware or plastic cloth) cage, which was located midway between the speakers. After each alternative stimulus had been played back several times in an alternating fashion, the top of the cage was removed remotely. Female movements were observed under infra-red illumination with a closed-circuit video system. A response was tabulated when the female showed phonotactic orientation behavior (Rheinlaender et al., 1979) and moved to within 10 cm of one of the speakers. If a female did not begin phonotaxis within 5 min or hopped away from the testing area within the chamber, the trial was recorded as a “no response.” Most responses occurred within 3 min of the female's release.
Each female provided a single datum for a test of a pair of alternatives at a given playback level. There was a minimum time-out of 2 min for females that were used in more than one test. Control experiments have demonstrated that female treefrogs (H. cinerea and H. versicolor) were not biased for or against a stimulus they heard or responded to in a previous two-stimulus test (Gerhardt, 1981; Gerhardt et al., 2000). Although the different alternatives were switched periodically between speakers within and between two-stimulus testing to minimize the effects of possible side biases, no such biases were detected (see also Gerhardt et al., 2000). Most females were later released at the site of capture or held in the animal care facilities and used for other studies. Facilities and experimental procedures were approved by the University of Missouri Animal Care and Use Committee.
Data analysis
Results are presented as the proportions of females that chose one of the alternatives (Figs 2-4). I computed the 95%-exact confidence limits using the F-distribution method employed in SAS Version 9.1. If a significant proportion chose one of the alternatives (two-tailed binomial, p < 0.05) occurred, I show only one-sided limits. However, because I did not pre-determine the sample size, such significance tests are not strictly valid, and I interpret the confidence limits as Bayesian credible intervals whose validity is independent of a stopping rule. Bayesian credible intervals correspond numerically to confidence limits when the prior distribution is uniform, which is also the assumption of classical significance tests (see Gerhardt, 1992 and references therein). Note that credible intervals are interpreted differently than confidence limits. The true proportion is assumed to have a 95%-probability of being within the 95%-credible limits, whereas if many confidence limits are computed, then 95% of them are expected to include the mean.
Figure 2.
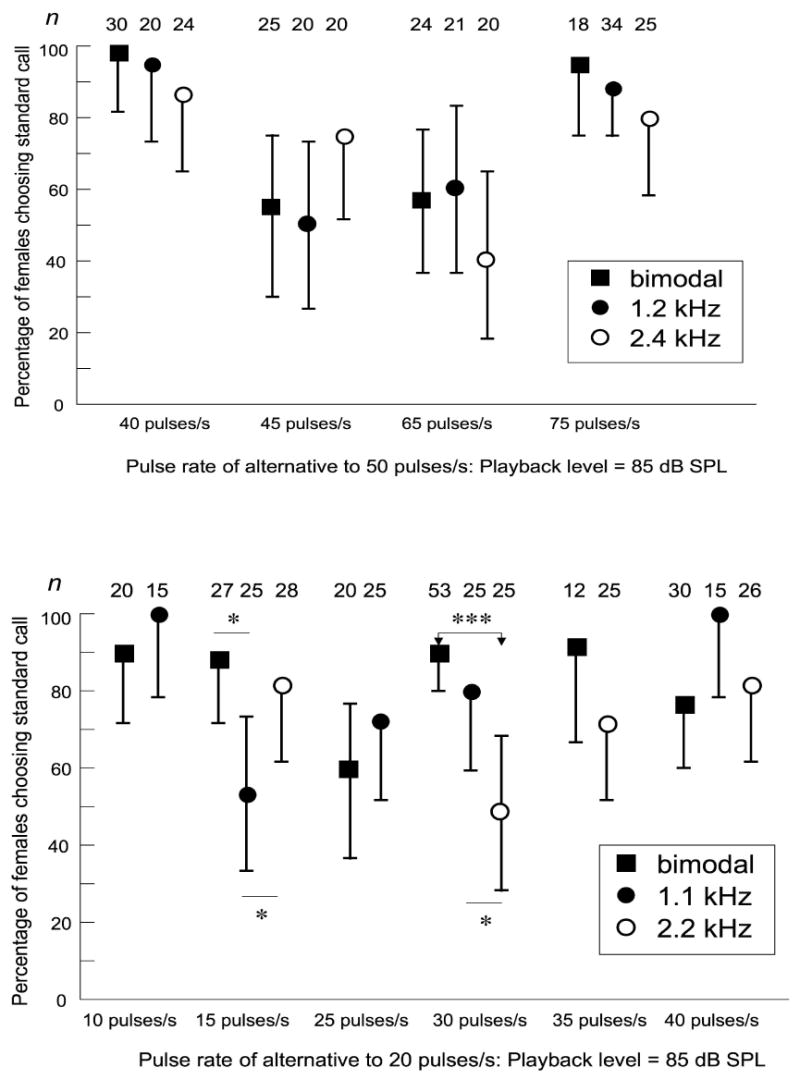
Comparisons of pulse-rate preferences using different carrier frequencies at a playback level of 85 dB SPL. Data for bimodal spectra were previously published in Gerhardt (2005a) and are re-plotted here. (a) Preferences of females of H. chrysoscelis; (b) Preferences of females of H. versicolor. Error bars are 95% credible intervals, and statistically significant, post-hoc comparisons of least-square-means between the proportions of females choosing the standard call depending on carrier frequency are indicated by asterisks (* = p < 0.05; *** = p < 0.001). See Methods and Results for details about the statistical analyses. n = number of female choices (one per female in each test).
Figure 4.
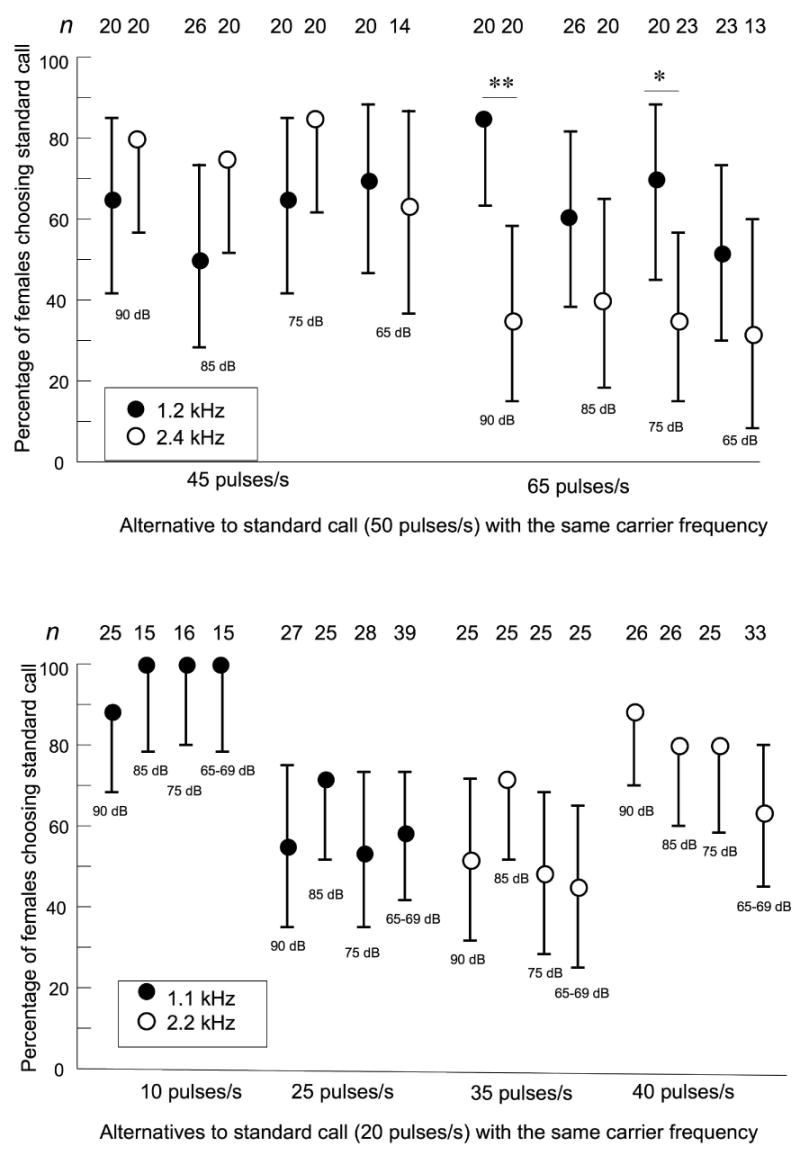
Intensity-dependence of pulse-rate preferences in tests between standard calls and alternatives with other pulse rates. (a) Preferences of H. chrysoscelis; pulse-rate differences were less than those between the standard call and alternatives at the low- and high-cutoff values determined with stimuli having a bimodal spectrum (Gerhardt 2005a). When these data were combined with those plotted in Fig. 3, there were highly significant effects of pulse rate. See text for details. (b) Preferences of H. versicolor in tests with an alternative with a lower pulse rate (10 pulses/s) than the lower cut-off value and with alternatives with higher pulse rates (35 pulses/s and 40 pulses/s) than the higher cut-off value. Error bars are 95% credible intervals. n = number of female choices (one per female in each test).
The main results were analyzed using the Genmod procedure (Statistical Analysis System Institute, v. 11.1, Cary, NC, USA), which uses maximum likelihood estimation to fit generalized linear models. I used a logit link function and binomial response function and report Type 3 likelihood ratios that have an asymptotic Chi-square distribution. These analyses used all of the data, with the assumption that each response was independent, even if a female contributed a response in more than one different test. Post-hoc comparisons were based on differences in least-square means or, in some tests of the intensity-dependence of choices (Fig. 4b), by log-likelihood tests of two-by-four tables.
Results
Females of H. chrysoscelis (n = 224 of 268 tested) responded in 636 of 1036 tests; females of H. versicolor (n = 495 of 649 tested) responded in 873 of 1734 tests. The proportion of responses was lowest in tests at low playback levels (48% in H. chrysoscelis and 35% in H. versicolor at 65 dB SPL) and highest at the playback level of 85 dB SPL (85% in H. chrysoscelis and 83% in H. versicolor). Nevertheless, simple inspection of the figures summarizing the results indicates that females were in general no less likely to choose the standard call at low playback levels than at moderate to high levels. In other words, playback level was related to the likelihood that a female would respond at all but did not predict selectivity.
Choices for standard calls with the species-typical bimodal spectrum (data from Gerhardt 2005b) are compared with those in tests using single-peaked calls in Fig. 2; the alternative stimuli were equalized at 85 dB SPL. In H. chrysoscelis carrier frequency had no significant effect on the proportions of females choosing the standard call of 50 pulses/s relative to any of the alternatives with other pulse rates (Main effect of frequency: Likelihood ratio [LR] chi-square = 2.47, df = 2, p = 0.29; interaction of frequency and pulse rate: LR chi-square = 7.58, df = 6, p = 0.27). In H. versicolor, the interaction between carrier frequency and the pulse rate of the alternative was highly significant (LR chi-square = 19.75, df = 7, p = 0.006). Post-hoc comparisons confirmed that a significantly higher proportion of females chose the standard call of 20 pulses/s than the alternative of 30 pulses/s when alternatives had a bimodal spectrum (Difference in least square means [DLSM]: Chi- square = 13.13, df = 1, p = 0.0003) or a carrier frequency of 1.1 kHz (DLSM: Chi- square = 5.24, df = 1, p = 0.022) than when the carrier frequency of alternatives was 2.2 kHz. Significantly higher proportions of females also chose the standard call with the bimodal carrier frequency (DLSM: chi-square = 5.98, df = 1, p = 0.015) or with the 2.2-kHz carrier frequency (DLSM: chi-square = 4.07, df = 1, p = 0.044) than than the alternative of 15 pulses/s when it had a carrier frequency of 1.1 kHz
Fig. 3 shows the results of tests in which pulse rate, carrier frequency, and playback level were systematically varied; pulse rates of alternatives had values that were less attractive than the standard call when both alternatives had a bimodal carrier and a playback level of 85 dB (Gerhardt 2005b). In H. chrysoscelis, the full model indicated that the only the significant main effect was carrier frequency (LR Chi-square = 24.85, df = 1, p < 0.0001). The general pattern seen in Fig. 3A, however, is that females were more selective for the standard call when the carrier frequency was 1.2 kHz rather than 2.4 kHz. Although the interaction effects were non-significant in this analysis and so no formal post-hoc test is justified, the greatest difference in selectivity occurred in tests against the alternative of 75 pulses/s at low SPLs. When two additional alternatives (45 pulses/s and 65 pulses/s) were included, there were also significant effects of pulse rate and the interaction of pulse rate and carrier frequency (see Fig. 4A and below).
Figure 3.
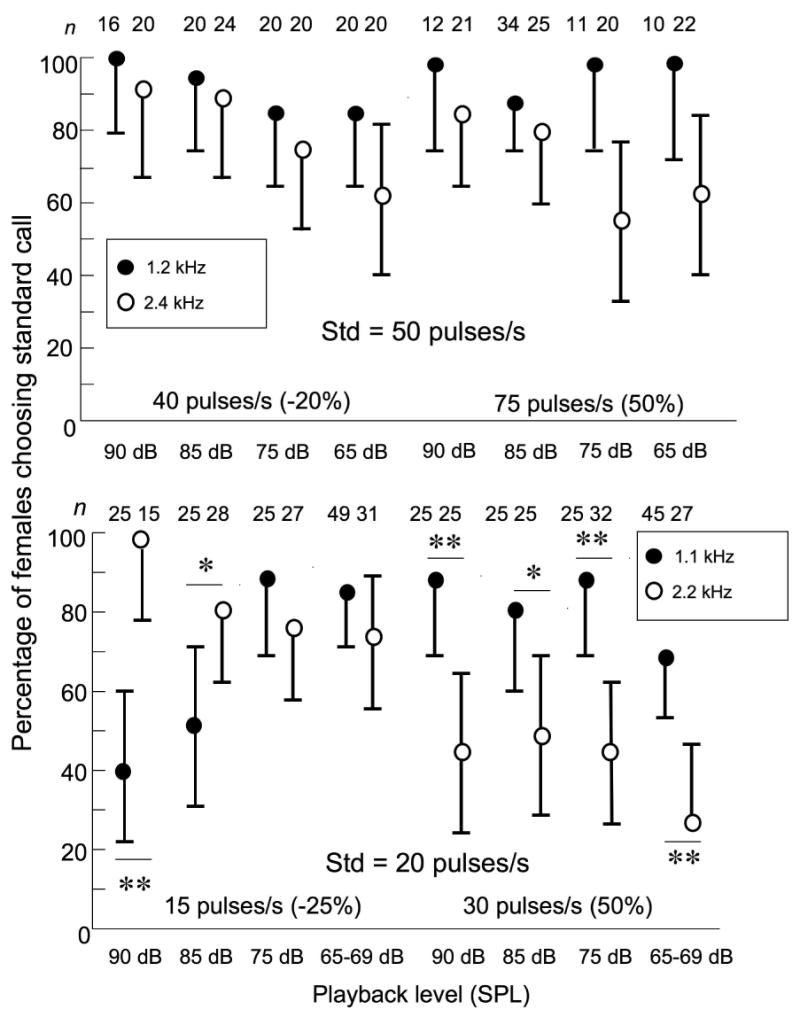
Intensity-dependence of pulse-rate preferences in tests between standard calls and alternatives with pulse rates at the low- and high-cutoff values (see text for definition). (a) Preferences of H. chrysoscelis; (b) Preferences of H. versicolor. Error bars are 95% credible intervals, and statistically significant, post-hoc comparisons of least-square-means between the proportions of females choosing the standard call depending on carrier frequency are indicated by asterisks (* = p < 0.05; ** = p < 0.01). See Methods and Results for details about the statistical analyses. n = number of female choices (one per female in each test).
In H. versicolor by contrast, there was a significant three-way interaction among the variables (pulse rate, carrier frequency, and playback level)(LR chi-square = 10.30, df = 3, p = 0.016). The main effects of pulse rate and carrier frequency and their interaction were also statistically significant as was the interaction between frequency and playback level. Post-hoc comparisons indicated that in all except two tests, there was a significant difference in the proportions of females choosing the alternative with the standard pulse rate that depended on carrier frequency (Fig. 3b shows the significance levels in terms of DSLMs). At all four playback levels, significant proportions of females chose the standard call of 20 pulses/s rather than the alternative of 30 pulses/s when the carrier frequency was 1.1 kHz but not when the carrier frequency was 2.2 kHz. When the alternative had a pulse rate of 15 pulses/s significant proportions of females chose the standard call when the carrier frequency was 2.2 kHz at all four playback levels; females showed no preference when the carrier frequency was 1.1 kHz at playback levels of 85 and 90 dB SPL.
Fig. 4a shows the choices of H. chrysoscelis in additional tests of alternatives with pulse-rate differences that were did not result in preferences for either alternative when tested with calls with bimodal spectra at a playback level of 85 dB SPL (Gerhardt 2005b). There was a significant effect of pulse rate (LR chi-square = 15.28, df = 2, p = 0.0005) and of the interaction between pulse rate and carrier frequency (LR chi-square = 8.67, df = 1, p = 0.003). When these data were combined with those plotted in Fig. 3A, there were also highly significant effects of pulse rate (LR chi-square 50.47, df = 3, p < 0.0001; carrier frequency (LR chi-square = 12.73, df = 1, p = 0.0004) and the interaction of these two variables (LR chi-square = 20.43, df = 3, p = 0.0001). Carrier-frequency effects were significant at pulse rates of 40 pulses/s (DLSM chi-square = 4.39, df = 1, p = 0.036), 65 pulses/s (DLSM chi-square = 14.75, df = 1, p = 0.0001), and 75 pulses/s (DLSM chi-square = 6.03, df = 1, p = 0.014), and there was a strong trend at 45 pulses/s (DLSM chi-square = 3.43, df = 1, p = 0.064). In summary, females were more likely to choose the standard call when the carrier frequency was 1.2 kHz rather than 2.4 k Hz and the pulse rate of the alternative was higher. The effect of carrier frequency was weak or absent when the pulse rate of the alternative was lower.
Fig. 4b shows the choices of H. versicolor in additional tests of alternatives with different pulse rates at all four playback levels. Because not all pulse-rate alternatives were tested with both carrier frequencies, the data were not analyzed with the genmod procedure. There were no statistically significant effects of playback level for any of the four sets of tests (p-values of log-likelihood estimates of 2 X 4 tables were all > 0.1). These results reinforce the fact that females showed poor selectivity over a wide range of playback levels when the carrier frequency was 2.2 kHz and alternatives had pulse rates even higher than 30 pulses/s. Only when the pulse rate of the alternative was increased to 40 pulses/s did significant proportions of females choose the standard call, and this choice was far from unanimous. By contrast, even though most females did not choose the standard call of 20 pulses/s with a carrier frequency of 1.1 kHz in preference to an alternative with the same carrier frequency and a pulse rate of 15 pulses/s at playback levels of 90 and 85 dB SPL, significantly higher proportions of females chose the standard call at all playback levels when the pulse rate of the alternative was reduced to 10 pulses/s.
Discussion
Patterns of preference
Female selectivity for pulse rates typical of conspecific calls often depended on which of the two spectral peaks typical of conspecific calls was present. There are two reasons to conclude that the effects of carrier frequency documented here are most likely to be attributable to differences in processing by the low- and high-frequency channels of the anuran auditory system rather than some subtle spectral differences that exist at different pulse rates, such as differences in sideband frequencies. First, although any change in a temporal property also affects the spectrum of nearly all signals, an exception is the long-term spectrum of broadband noise, which is independent of amplitude modulation (Rice 1955). I exploited this characteristic of noise to show that in another species of North American treefrog (H. cinerea), females discriminated between unmodulated and pulsed (50 pulses/s) signals (noise bursts) based on the difference in the amplitude-time waveform alone (Gerhardt 1978). Second, many auditory neurons in both the low- and high-frequency channels of the anuran auditory system show reliable encoding (phase locking to each pulse in a train of pulses) of pulse rates higher than those of any stimulus used in these experiments (Rose and Gooler, 2006).
Temporal selectivity was usually greater when the carrier frequency of both alternatives was low (1.1 or 1.2 kHz) rather than high (2.2 or 2.4 kHz). Effects were much less pronounced in H. chrysoscelis, in which selectivity was comparable to that occurring in experiments in which both spectral peaks were present (Gerhardt 2005b) than in H. versicolor, In the latter species there were significant interactions among carrier frequency, the direction of the pulse-rate difference vis-à-vis that of the standard call, and playback level. Females of H. versicolor were more selective than females of H. chrysoscelis in a few tests when the high-frequency peak was used and the playback level was high.
At 85 dB SPL, which corresponds to a distance of 1-2 m from a typical calling male and to the playback level that has been mostly commonly used in studies of these species, females of H. chrysoscelis showed the same selectivity for pulse-rate differences regardless of whether the carrier frequency was 1.2 kHz, 2.4 kHz or the usual bimodal spectrum (Fig. 2a). Generalizing these results by varying the playback level, however, indicated that temporal selectivity was sometimes weaker or absent at lower playback levels when the 2.4-kHz carrier was used (Figs. 3 and 4). By contrast, when the carrier frequency of both alternatives was 1.2 kHz, more than 80% of the females chose the standard call of 50 pulses/s over alternatives with pulse rates of 40 pulses/s and 75 pulses/s at all four playback levels (Fig. 3a). Females even preferred the standard call to an alternative of 65 pulses/s at playback levels of 90 and 75 dB SPL (Fig. 4a).
In H. versicolor, striking and complex effects of carrier frequency on the temporal preferences of females were already evident in the initial tests at 85 dB SPL (Fig. 2b). Females failed to discriminate against an alternative of 30 pulses/s when the carrier frequency was 2.2 kHz. This result is surprising because this is the dominant frequency band in their advertisement call, and females preferred an alternative with a 2.2-kHz carrier frequency to an alternative with a carrier frequency of 1.1 kHz at 85 dB SPL when both alternatives had the standard pulse rate of 20 pulses/s (Gerhardt, 2005a). Furthermore, the alternative pulse rate of 30 pulses/s falls within the range of variation of H. chrysoscelis, a genetically incompatible species that often breeds at the same time and place. The absence of a preference for the standard call was also observed at the three other playback levels (Figs 3b), and other tests showed that discrimination against alternatives of 35 and 40 pulses/s was also weak or absent when the carrier frequency was 2.2 kHz (Fig. 4b).
A second unexpected result seemingly runs counter to the previous indication that selectivity was generally high when the low-frequency carrier was used. That is, there was no preference for the standard call when the carrier frequency was 1.1 kHz and the alternative had a pulse rate of 15 pulses/s at 85 and 90 dB SPL (Figs. 2b and 3b). Strong preferences for the standard call did occur, however, at playback levels of 65 and 75 dB SPL in the same test, and females chose the standard call at all playback levels when the pulse rate was decreased to 10 pulses/s (Fig. 4b).
Interpretation and hypotheses
The general superiority of the low-frequency peak in mediating phonotactic selectivity based on fine-scale temporal properties was anticipated by a previous study of pulse-shape selectivity in H. versicolor (Gerhardt and Schul, 1999). When pulse rate was held constant at 20 pulses/s (25 ms pulse duration and 25 ms interpulse interval, as in the standard call used in the present study), females discriminated between calls with a rise-time difference of 5 ms when the carrier frequency was either 1.1 or 2.2 kHz at a playback level of 85 dB SPL. However, when the playback level was lowered to 75 dB SPL, females discriminated only when the low-frequency carrier was used. Gerhardt and Schul (1999) hypothesized that pulse-rise time selectivity is accomplished by the low-frequency channel and that preferences observed at 85 dB SPL with the 2.2 kHz carrier frequency occurred because of cross-talk. That is, at such high levels, substantial numbers of receptors and auditory neurons in the low-frequency channel would also be excited. The same kind of argument can be used to explain patterns of intensity-dependent selectivity of females of H. chrysoscelis in tests of alternatives with higher pulse rates than that of standard call. Alternatively, neurons in the high-frequency channel may be generally less sensitive than those in the low-frequency channel and hence require higher stimulus levels in order to resolve fine-scale time differences. The underlying mechanism could reflect, in part, the better phase-locking ability of auditory-nerve fibers innervating the amphibian papilla (low-frequency-tuned inner ear organ) than auditory-nerve fibers innervating the basilar papilla (high-frequency-tuned inner ear organ)(Dunia and Narins, 1989; Simmons et al., 1993), although even high-frequency-tuned fibers should still be able to reliably phase-lock to pulses repeated at rates of 75 pulses/s or less.
An explanation based on frequency-dependent phase-locking and cross-talk between the two inner ear organs is, however, inadequate to explain the pattern of selectivity shown by females of H. versicolor. First, when the high-frequency carrier was used there was no improvement in selectivity in tests of alternatives of higher-than-standard pulse rates when the playback level was increased to 85 and 90 dB SPL. Second, females did not prefer the standard call when the carrier frequency was 1.1 kHz and the alternative was 15 pulses/s at playback levels of 90 and 85 dB SPL. I now re-emphasize that because pulse duty-cycle (ratio of pulse duration to pulse period) was held constant in the present study (the natural pattern in the calls of both species), the absolute values of pulse duration and pulse intervals were longer in synthetic calls with pulse rates lower than the standard pulse rate; the values of these properties were shorter in synthetic calls with pulse rates higher than the standard (see Fig. 1). It follows that the absolute values of pulse duration and intervals were longer in the majority of stimuli presented to H. versicolor than those presented to H. chrysoscelis. Another variable that differed between species was the shape and rise-time of the pulses, which affects the selectivity of females of H. versicolor (Diekamp and Gerhardt, 1995; Gerhardt and Schul, 1999). Females of H. chrysoscelis were unselective with regard to species-specific pulse shape differences over the normal range of variation in pulse duration but did prefer stimuli with the conspecific pulse shape when the duration of pulses was increased so that the absolute rise-time differences were comparable to those discriminated by H. versicolor (Gerhardt 2005b).
My speculative explanation for the puzzling results in H. versicolor is based on the acoustic differences just discussed, species differences in the acoustic criteria whereby females evaluate pulsed calls, and on recent studies of how different classes of temporally selective neurons respond to different kinds of pulsed signals. Females of H. chrysoscelis use pulse-rate (largely independent of pulse duty cycle) as the main criterion for fine-scale temporal discrimination (Schul and Bush, 2002). Female selectivity is well correlated with the response properties of a class of auditory neurons found in the anuran torus semicircularis (midbrain) that show sharp band-pass selectivity for relatively fast rates of amplitude modulation (Rose and Gooler, 2006). These integration-type neurons require some minimum number of correct interpulse intervals, do not synchronize to the pulses within a stimulus, and are relatively unaffected by duty cycle (pulse duration) (Adler and Rose, 2000).
Females of H. versicolor require both a minimum pulse duration and maximum silent interval (Schul and Bush, 2002). In other words, their temporal selectivity is not based on pulse rate per se. Thus, fine-scale pattern recognition in H. versicolor might be limited at least in part by how well primary auditory neurons and tonic-firing, primary-like neurons in ascending pathway encode pulse duration rather than by how well phasic neurons synchronize to each pulse (review Rose and Gooler, 2006). Indeed, a subset of about 20% of primary-like neurons in the torus semicircularis of three anuran species is duration selective (review: Rose and Gooler, 2006). Other duration-selective neurons respond only when pulses exceed some minimum duration or when some minimum number of shorter pulses are rapidly repeated (Leary et al., 2008). Such “long-pass” neurons, therefore, show integrating (interval counting) properties (Edwards et al., 2005) and could also play a role over some ranges of pulse duration and rise-time. Whatever the mechanism, it must explain the poor and intensity-independent selectivity observed when the high-frequency carrier was used and the alternatives had a higher pulse rate and the even more problematic, intensity-dependent selectivity observed when the low-frequency carrier was used and the alternatives had a pulse rate of 15 pulses/s. Long-interval selective neurons may be involved in the latter phenomenon. At SPLs substantially above threshold the dominance of inhibition can decrease the response levels of temporally selective neurons (Rose, pers. comm.). Thus, the decreased behavioral selectivity at high amplitudes and low carrier frequency might have resulted from an inhibition-related attenuation of responses to the 20 pulses/s (standard) stimulus, which was more attractive at lower SPLs.
General mechanisms of temporal selectivity and future directions
One emerging perspective is that a wide variety of mechanisms that underlie temporal selectivity probably depend on within-cell interactions between inhibition and excitation rather than, or in addition to, convergence of the outputs of lower-order neurons with various filtering processes (e.g., Casseday et al. 2000; Edwards et al. 2007; Bush and Schul 2005). Moreover, neural modeling suggests that, in general, single neurons can be expected to respond in fundamentally different ways to pulse trains with different values of pulse duration and intervals (e.g., Izhikevich 2001).
Given the diversity of potential auditory mechanisms, it is perhaps not surprising that a species like H. versicolor shows such complex patterns of frequency- and intensity-dependent preferences. Not only do females have to deal with a wide range of pulse rate (9 to 32 pulses/s) and pulse duration (15-55 ms) found in conspecific calls over the normal range of breeding temperatures (Gayou, 1984), but also the longer absolute durations of pulses are likely to engage auditory neurons that selectively respond to pulses that meet or exceed these durations and rise-time. The closely related bird-voiced treefrog (Hyla avivoca) produces calls with pulses and interpulse intervals that are even longer than those in the calls of H. versicolor. The results of preliminary tests indicate that the fine-scale temporal criteria of females of H. avivoca also involve assessments of pulse duration and intervals rather than pulse rate per se (Martínez and Gerhardt, unpubl. data). Moreover, unlike the gray treefrogs the advertisement call of this species has single spectral peak of relatively high frequency (Gerhardt, 2001). Could it be that a shift from low-frequency or high-frequency mediated, pulse-rate discrimination (as shown by H. chrysoscelis) to primarily high-frequency mediated, pulse-duration discrimination occurs in species that must correctly identify signals with relatively long pulses and different amplitude-time envelopes? The pulse-duration dependence of pulse shape by females of H. chrysoscelis also supports this concept. Females did not prefer pulses with the conspecific pulse shape unless pulse duration was increased to values greater than those produced by conspecific males so that rise-time differences became comparable to those discrimination by females of H. versicolor (Gerhardt, 2005b). As pointed out by Adler and Rose (2000), neurons with the potential for dealing with a variety of pulse forms and rates probably occur in most species, and other models, such as those involving resonance mechanisms, indicate that dramatic differences in response properties can be achieved by small changes in membrane potential (Bush and Schul, 2005).
In any event, the results of the present study suggest that the carrier frequency of the temporal information is another factor that should be considered in studies of the neural mechanisms of fine-scale temporal selectivity. For example, are there any biases in the frequency tuning of recovery-type, integrative-type and duration-selective cells in the frog torus semicircularis that might also depend on the pulse rates (interpulse intervals) to which they are most selective? Finally, changes in intensity had non-linear effects on frequency preferences in the two species of gray treefrogs (Gerhardt et al., 2007), and so it is hardly surprising that many of the choices demonstrated in this paper were also intensity-dependent. Another task for behavioral biologists is to generalize their results over a reasonably wide range of sound intensities because only by doing so can we provide a useful set of specifications for studies of auditory mechanisms as well as gaining insights about how these animals recognize biologically important sounds in their natural environment.
Acknowledgments
I thank S. Bisges, A. Bockhorst, T. Cook, D. Dittmer, A. Evers, N. Gordon, M. Hellman, C. Hoai, , G. Höbel, K. Huth, N. Maximiv, V. Marshall, C. McConkey, A. Miller, C. Plakosh, C. Tegtmeyer and M. Tucker for assistance in collecting and testing frogs. G. Rose , J. Schul, N. Gordon, S. Humfeld and two anonymous reviewers provided helpful comments on the manuscript. S. Humfeld provided general technical assistance. This work was supported by the National Science Foundation (IBN-0091993) and the Public Health Service (DHHS R01 DC05760 to Gerhardt).
References
- Alder TB, Rose GJ. Integration and recovery processes contribute to the temporal selectivity of neurons in the midbrain of the northern leopard frog, Rana pipiens. J Comp Physiol A. 2000;186:923–937. doi: 10.1007/s003590000144. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Bush SL, Schul J. Pulse-rate recognition in an insect: evidence for a role of oscillatory neurons. J Comp Physiol A. 2005;192:113–121. doi: 10.1007/s00359-005-0053-x. [DOI] [PubMed] [Google Scholar]
- Capranica RR, Moffat AJM. Neurobehavioral correlates of sound communication in anurans. In: Ewert JP, Capranica RR, Ingle DJ, editors. Advances in Vertebrate Neuroethology. New York: Plenum Press; 1983. pp. 701–730. [Google Scholar]
- Casseday JH, Ehrlich D, Covey E. Neural measurements of sound duration: control by excitatory-inhibitory interactions in the inferior colliculus. J Neurophysiol. 2000;84:1475–1487. doi: 10.1152/jn.2000.84.3.1475. [DOI] [PubMed] [Google Scholar]
- Diekamp BM, Gerhardt HC. Selective phonotaxis to advertisement calls in the gray treefrog, Hyla versicolor: Behavioral experiments and neurophysiological correlates. J Comp Physiol A. 1995;177:173–190. doi: 10.1007/BF00225097. [DOI] [PubMed] [Google Scholar]
- Dunia R, Narins PM. Temporal resolution in frog auditory-nerve fibers. J Acoust Soc Am. 1989;85:1630–1638. doi: 10.1121/1.397951. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Alder TB, Rose GJ. Pulse rise time but not duty cycle affects the temporal selectivity of neurons in the anuran midbrain that prefer slow AM rates. J Neurophysiol. 2005;93:1336–1341. doi: 10.1152/jn.00797.2004. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Leary CJ, Rose GJ. Counting on inhibition and rate-dependent excitation in the auditory system. J Neurosci. 2007;27:13384–13392. doi: 10.1523/JNEUROSCI.2816-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayou DC. Effects of temperature on the mating call of Hyla versicolor. Copeia. 1984;1984:733–738. [Google Scholar]
- Gerhardt HC. Mating call recognition in the green treefrog (Hyla cinerea) significance of some fine-temporal properties. J Exp Biol. 1978a;74:59–73. doi: 10.1242/jeb.61.1.229. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Temperature coupling in the vocal communication system of the gray treefrog Hyla versicolor. Science. 1978b;199:992–994. doi: 10.1126/science.199.4332.992. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Mating call recognition in the green treefrog (Hyla cinerea): importance of two frequency bands as a function of sound pressure level. J Comp Physiol A. 1981;144:9–16. [Google Scholar]
- Gerhardt HC. Conducting playback experiments and interpreting their results. In: MacGregor P, editor. Playback and Studies of Animal Communication: Problems and Prospects. New York: Plenum Press; 1992. pp. 59–77. [Google Scholar]
- Gerhardt HC. Reproductive character displacement of female mate choice in the grey treefrog H. chrysoscelis. Anim Behav. 1994;47:959–969. [Google Scholar]
- Gerhardt HC. Acoustic communication in two groups of closely related treefrogs. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ, editors. Advances in the Study of Behavior. New York: Academic Press; 2001. pp. 99–167. [Google Scholar]
- Gerhardt HC. Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim Behav. 2005a;70:39–49. [Google Scholar]
- Gerhardt HC. Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution. 2005b;59:395–408. [PubMed] [Google Scholar]
- Gerhardt HC, Doherty JA. Acoustic communication in the gray treefrog, Hyla versicolor: evolutionary and neurobiological implications. J Comp Physiol A. 1988;162:261–278. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Frogs: Common Problems and Diverse Solutions. Chicago: University of Chicago Press; 2002. [Google Scholar]
- Gerhardt HC, Schul J. A quantitative analysis of behavioral selectivity for pulse-rise time in the gray treefrog, Hyla versicolor. J Comp Physiol A. 1999;185:33–40. doi: 10.1007/s003590050363. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Schwartz JJ. Auditory tuning and frequency preferences in Anurans. In: Ryan MJ, editor. Anuran Communication. Washington: Smithsonian Institution Press; 2001. pp. 73–85. [Google Scholar]
- Gerhardt HC, Tanner SD, Corrigan CM, Walton HC. Female preference functions based on call duration in the gray treefrog (Hyla versicolor) Behav Ecol. 2000;11:663–669. [Google Scholar]
- Holloway AK, Cannatella DC, Gerhardt HC, Hillis DM. Polyploids with different origins and ancestors form a single polyploidy species. Am Nat. 2006;167:E88–101. doi: 10.1086/501079. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Resonate-and-fire neurons. Neural Networks. 2001;14:883–894. doi: 10.1016/s0893-6080(01)00078-8. [DOI] [PubMed] [Google Scholar]
- Leary CJ, Ewards CJ, Rose GJ. Midbrain auditory neurons integrate excitation and inhibition to generate duration selectivity: an, in vivo, whole-cell patch study in anurans. J Neurosci. 2008;28:5481–5493. doi: 10.1523/JNEUROSCI.5041-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narins PM, Feng AS, Fay RR, Popper AN. Hearing and Sound Communication in Amphibians. New York: Springer Science+Business Media; 2006. [Google Scholar]
- Ptacek MB, Gerhardt HC, Sage RD. Speciation by polyploidy in treefrogs: multiple origins of the tetraploid, Hyla versicolor. Evolution. 1994;48:898–908. doi: 10.1111/j.1558-5646.1994.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Rheinlaender J, Gerhardt HC, Yager D, Capranica RR. Accuracy of phonotaxis in the green treefrog (Hyla cinerea) J Comp Physiol. 1979;133:247–255. [Google Scholar]
- Rice EO. Mathematical analysis of random noise. In: Was N, editor. Selected Papers on Noise and Stochastic Processes. New York: Dover Press; 1955. [Google Scholar]
- Rose GJ, Gooler DM. Function of the amphibian central auditory system. In: Narins PM, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians. New York: Springer Science+Business Media); 2006. pp. 250–290. [Google Scholar]
- Schmidt A, Ronacher B, Hennig RM. The role of frequency, phase and time for processing of amplitude modulated signals by grasshoppers. J Comp Physiol A. 2007;194:221–233. doi: 10.1007/s00359-007-0295-x. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Meenerink WF, Vassilakis PN. Anatomy, physiology, and function of auditory end-organs in the frog inner ear. In: Narins AS, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians. New York: Springer Science+Business Media); 2006. pp. 184–220. [Google Scholar]
- Simmons AM, Reese G, Ferragamo M. Periodicity extraction in the anuran auditory nerve. II: Phase and temporal fine structure. J Acoust Soc Am. 1993;93:3374–3389. doi: 10.1121/1.405693. [DOI] [PubMed] [Google Scholar]
- Schul J, Bush SL. Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc R Soc London B. 2002;269:1847–1852. doi: 10.1098/rspb.2002.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkowiak W. Neuronal correlates of the recognition of pulsed sound signals in the grass frog. J Comp Physiol A. 1984;155:57–66. [Google Scholar]


