Abstract
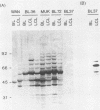
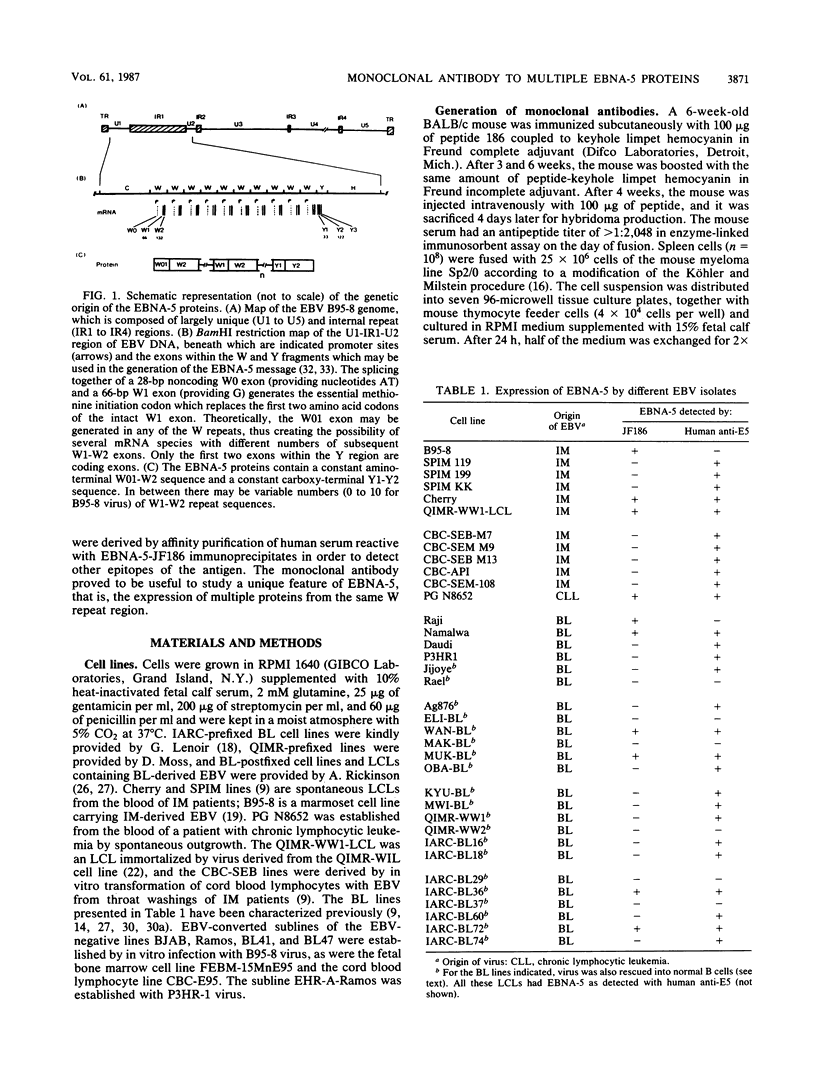
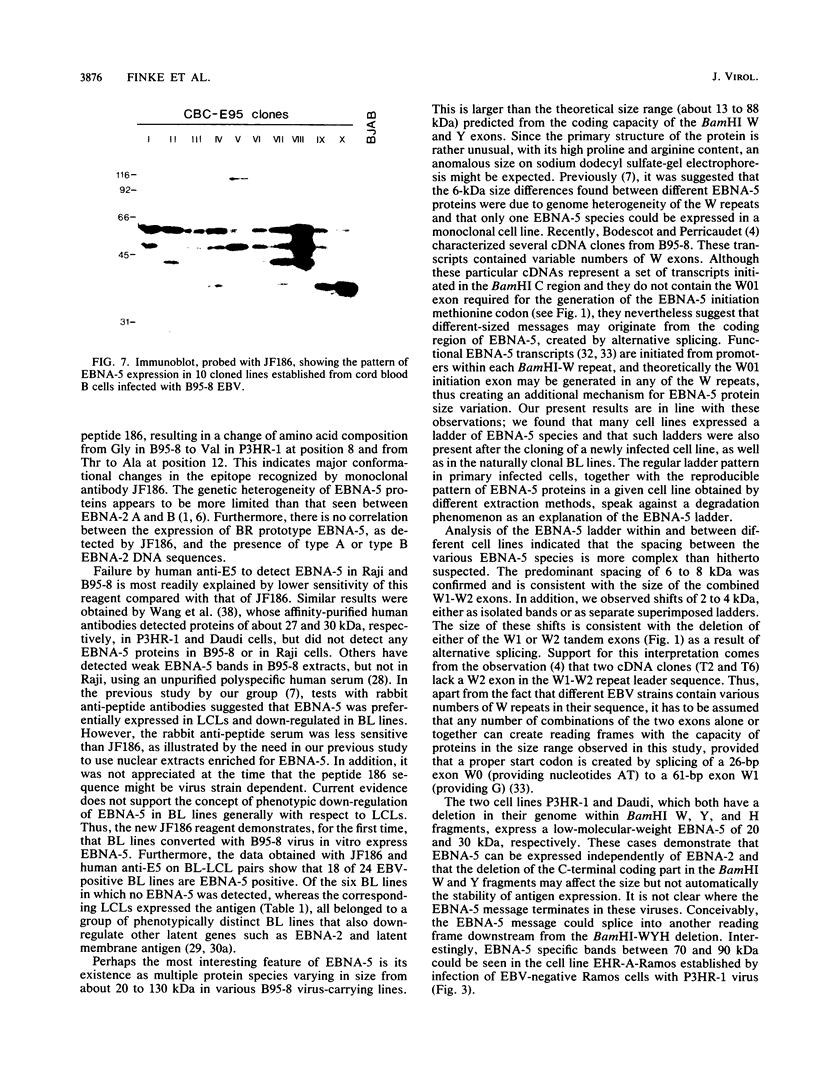
The Epstein-Barr virus nuclear antigen 5 (EBNA-5) is encoded by highly spliced mRNA from the major IR1 (BamHI-W) repeat region of the virus genome. A mouse monoclonal antibody, JF186, has been raised against a synthetic 18-amino-acid peptide deduced from the EBNA-5 message of B95-8 and Raji cells. The antibody showed characteristic coarse nuclear granules by indirect immunofluorescence and revealed multiple EBNA-5 species by immunoblotting and immunoprecipitation. The B95-8 line itself and all B95-8 virus-carrying cells, whether lymphoblastoid cell lines or in vitro-converted sublines of Epstein-Barr virus (EBV)-negative Burkitt's lymphoma (BL) lines, were EBNA-5 positive. Among 36 cell lines carrying different EBV strains, only 10 expressed the B95-8-Raji-prototype EBNA-5 recognized by JF186; this was probably due to genetic variation in the epitope recognized by JF186, as shown for P3HR-1. Human antibodies, affinity purified against EBNA-5-JF186 immunoprecipitates, detected EBNA-5 in the majority of EBV-positive BL lines and in all lymphoblastoid cell lines containing the BL-derived viruses. Thus, EBNA-5 can be expressed by all virus isolates examined, but is down-regulated, together with other latent gene products, in a minority of BL lines which have a particular cellular phenotype. EBNA-5 was detected as a ladder of protein species of 20 to 130 kilodaltons (kDa), with a regular spacing of 6 to 8 kDa, consistent with the coding capacity of the combined BamHI-W 66- and 132-base-pair exons, together with shifts of 2 to 4 kDa, consistent with the size of the separate 66- and 132-base-pair exons. Multiple EBNA-5 proteins can be expressed by the single cell as shown by cloning of newly infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adldinger H. K., Delius H., Freese U. K., Clarke J., Bornkamm G. W. A putative transforming gene of Jijoye virus differs from that of Epstein-Barr virus prototypes. Virology. 1985 Mar;141(2):221–234. doi: 10.1016/0042-6822(85)90253-3. [DOI] [PubMed] [Google Scholar]
- Bodescot M., Brison O., Perricaudet M. An Epstein-Barr virus transcription unit is at least 84 kilobases long. Nucleic Acids Res. 1986 Mar 25;14(6):2611–2620. doi: 10.1093/nar/14.6.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodescot M., Chambraud B., Farrell P., Perricaudet M. Spliced RNA from the IR1-U2 region of Epstein-Barr virus: presence of an open reading frame for a repetitive polypeptide. EMBO J. 1984 Aug;3(8):1913–1917. doi: 10.1002/j.1460-2075.1984.tb02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodescot M., Perricaudet M. Epstein-Barr virus mRNAs produced by alternative splicing. Nucleic Acids Res. 1986 Sep 11;14(17):7103–7114. doi: 10.1093/nar/14.17.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Bornkamm G. W. Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol. 1978 Jul;27(1):81–89. doi: 10.1128/jvi.27.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Kallin B., Alexander H., Ernberg I., Uno M., Ono Y., Klein G., Lerner R. A. An Epstein-Barr virus (EBV)-determined nuclear antigen (EBNA5) partly encoded by the transformation-associated Bam WYH region of EBV DNA: preferential expression in lymphoblastoid cell lines. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6641–6645. doi: 10.1073/pnas.83.17.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Kallin B. The Epstein-Barr virus proteins. Adv Cancer Res. 1988;50:95–158. doi: 10.1016/s0065-230x(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Ernberg I., Falk K., Hansson M. Progenitor and pre-B lymphocytes transformed by Epstein-Barr virus. Int J Cancer. 1987 Feb 15;39(2):190–197. doi: 10.1002/ijc.2910390212. [DOI] [PubMed] [Google Scholar]
- Ernberg I., Kallin B., Dillner J., Falk K., Ehlin-Henriksson B., Hammarskjöld M. L., Klein G. Lymphoblastoid cell lines and Burkitt-lymphoma-derived cell lines differ in the expression of a second Epstein-Barr virus encoded nuclear antigen. Int J Cancer. 1986 Nov 15;38(5):729–737. doi: 10.1002/ijc.2910380517. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Kieff E. A second nuclear protein is encoded by Epstein-Barr virus in latent infection. Science. 1985 Mar 8;227(4691):1238–1240. doi: 10.1126/science.2983420. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Wang F., Bushman E. W., Kieff E. Definitive identification of a member of the Epstein-Barr virus nuclear protein 3 family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5693–5697. doi: 10.1073/pnas.83.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson H. B., Farrell P. J., Miller G. Sequences of the Epstein-Barr Virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J Virol. 1987 May;61(5):1495–1506. doi: 10.1128/jvi.61.5.1495-1506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. D., Foster L., Sheedy T., Griffin B. E. The EB virus genome in Daudi Burkitt's lymphoma cells has a deletion similar to that observed in a non-transforming strain (P3HR-1) of the virus. EMBO J. 1984 Apr;3(4):813–821. doi: 10.1002/j.1460-2075.1984.tb01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Dillner J., Ernberg I., Ehlin-Henriksson B., Rosén A., Henle W., Henle G., Klein G. Four virally determined nuclear antigens are expressed in Epstein-Barr virus-transformed cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1499–1503. doi: 10.1073/pnas.83.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Hennessy K., Fennewald S., Matsuo T., Dambaugh T., Heller M., Hummel M. Biochemistry of latent Epstein-Barr virus infection and associated cell growth transformation. IARC Sci Publ. 1985;(60):323–339. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenoir G. M., Vuillaume M., Bonnardel C. The use of lymphomatous and lymphoblastoid cell lines in the study of Burkitt's lymphoma. IARC Sci Publ. 1985;(60):309–318. [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. H. Establishment of cell lines from Australian leukaemic patients: presence of a herpes-like virus. Aust J Exp Biol Med Sci. 1968 Oct;46(5):643–645. doi: 10.1038/icb.1968.171. [DOI] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Rumpold H., Kurki P., Patrick K. M., Carson D. A., Vaughan J. H. Autoantibodies in infectious mononucleosis have specificity for the glycine-alanine repeating region of the Epstein-Barr virus nuclear antigen. J Exp Med. 1987 Apr 1;165(4):1026–1040. doi: 10.1084/jem.165.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Young L. S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987 May;61(5):1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C. M., Gregory C. D., Rowe M., Finerty S., Edwards C., Rupani H., Rickinson A. B. Endemic Burkitt's lymphoma: phenotypic analysis of tumor biopsy cells and of derived tumor cell lines. J Natl Cancer Inst. 1986 Sep;77(3):681–687. doi: 10.1093/jnci/77.3.681. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Farrell P. J., Miller G. Novel nuclear antigens recognized by human sera in lymphocytes latently infected by Epstein-Barr virus. Virology. 1987 Jan;156(1):153–162. doi: 10.1016/0042-6822(87)90446-6. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Rowe M., Evan G. I., Wallace L. E., Farrell P. J., Rickinson A. B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J. 1986 Oct;5(10):2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rooney C. M., Edwards C. F., Lenoir G. M., Rickinson A. B. Epstein-Barr virus status and tumour cell phenotype in sporadic Burkitt's lymphoma. Int J Cancer. 1986 Mar 15;37(3):367–373. doi: 10.1002/ijc.2910370307. [DOI] [PubMed] [Google Scholar]
- Rymo L., Klein G., Ricksten A. Expression of a second Epstein-Barr virus-determined nuclear antigen in mouse cells after gene transfer with a cloned fragment of the viral genome. Proc Natl Acad Sci U S A. 1985 May;82(10):3435–3439. doi: 10.1073/pnas.82.10.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Pfitzner A., Strominger J. L. An Epstein-Barr virus transcript from a latently infected, growth-transformed B-cell line encodes a highly repetitive polypeptide. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9298–9302. doi: 10.1073/pnas.83.24.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Strominger J. L. Analysis of the transcript encoding the latent Epstein-Barr virus nuclear antigen I: a potentially polycistronic message generated by long-range splicing of several exons. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8305–8309. doi: 10.1073/pnas.82.24.8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T. C., Hopkins R. F., 3rd, Neubauer R. H., Rabin H. Identification of an Epstein-Barr virus nuclear antigen by fluoroimmunoelectrophoresis and radioimmunoelectrophoresis. J Virol. 1981 Jun;38(3):996–1004. doi: 10.1128/jvi.38.3.996-1004.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Grogan E. A., Shedd D., Robert M., Liu C. R., Miller G. Stable expression in mouse cells of nuclear neoantigen after transfer of a 3.4-megadalton cloned fragment of Epstein-Barr virus DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5688–5692. doi: 10.1073/pnas.79.18.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Petti L., Braun D., Seung S., Kieff E. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J Virol. 1987 Apr;61(4):945–954. doi: 10.1128/jvi.61.4.945-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]