Abstract
Entomological surveys in urban areas are often biased by selecting houses or locations with known high vector densities. A sampling strategy was developed for Puntarenas, Costa Rica, using high-resolution satellite imagery. Grids from the Advanced Spaceborne Thermal Emission and Reflection Radiometer and a QuickBird classified land cover map were used to determine the optimal final grid area for surveys. A random sample (10% of cells) was selected, and sample suitability was assessed by comparing the mean percentage of tree cover between sample and total cells. Sample cells were used obtain entomological data from 581 locations: 26.3% of all locations positive for mosquito larvae were not households, they contained 29.5% of mosquito-positive habitats and 16% of Aedes aegypti pupae collected. Entomological indices for Ae. aegypti (pupae per person, Breteau index, container index, location index) were slightly lower when only household data were analyzed. High-resolution satellite imagery and geographical information systems appear useful for evaluating urban sites and randomly selecting locations for accurate entomological surveys.
Keywords: Remote sensing, urban environment, mosquitoes, Aedes aegypti, Costa Rica
INTRODUCTION
Field evaluations for studying the epidemiology of mosquito-borne diseases in urban areas are commonly performed in locations where densities of mosquitoes and their habitats are known to be high. In addition, surveys are often restricted to sampling of households and buildings during surveys (Morrison et al. 2004, Chadee 2003). In Aedes aegypti surveillance, houses are usually sampled during pupal/demographic surveys, and houses are a main component of two traditional larval indices: House (or Premises) index (HI) and Breteau index (BI) (Focks and Chadee 1997, Focks 2003, Chadee 2004). In all cases, the resulting sampling frame may exclude locations within the complex urban environment such as streets, public buildings, parks, and schools that may provide valuable information about mosquito diversity and types of larval habitats. Therefore, in the case of diseases that are usually considered “urban” like dengue fever and dengue hemorrhagic fever, productive habitats may be overlooked during standard household surveys and bias the results. Sampling strategies for selecting mosquito collection sites may need to include non-residential locations in field surveys, such as those required for studying dengue and other vector-borne diseases of urban environments (Morrison et al. 2006, Barrera et al. 2006).
Geographical information systems (GIS) and remote sensing offer powerful tools for describing, illustrating, explaining, and predicting epidemiological phenomena, which can be used to develop or improve surveillance, prevention, and control strategies (Rogers and Randolph 2003). However, these technologies have been used to study vector-borne diseases mostly in non-urban areas and at very broad scales (Hay et al. 1997, Hay et al. 2000, Beck et al. 2000, Rogers et al. 2006). Data currently available from sensors like the Advanced Spaceborne Thermal Emission and Reflection Radiometer (ASTER, 15 m spatial resolution) and QuickBird (0.6 m panchromatic and 2.4 m multispectral spatial resolution) are useful for studying factors that affect diseases within the heterogeneous urban environment. In this report, a sampling strategy is described for the Great Puntarenas area, Costa Rica. This method was developed for sampling specific mosquito larval habitats using GIS technology and high-resolution satellite imagery from ASTER and QuickBird.
MATERIALS AND METHODS
The study site included ten localities of the Greater Puntarenas area, a city on the Pacific coast of Costa Rica where dengue fever is currently endemic. Puntarenas is the site of dengue reintroduction to Costa Rica in 1993 (WHO 1994), and no detailed entomological or georeferenced data in the form of GIS layers were available at the beginning of this study. High-resolution satellite images were obtained for the Greater Puntarenas area to develop the sampling strategy. Only two QuickBird scenes from March 2002 (dry season) and October 2003 (wet season) were available at very high resolution, each including a different section of the study site. Multispectral bands (blue, green, red, and near infrared) and the panchromatic band were obtained. In addition, ASTER imagery was available for those same years. All the GIS operations were performed using Idrisi Kilimanjaro software (J.R. Eastman, Clark University, Worcester, MA. 2004).
A classified land cover map generated from the mosaicked 2002 and 2003 multispectral QuickBird imagery by using the back propagation artificial neural network (ANN) in Idrisi Kilimanjaro was selected for the analyses. Training sites for “water”, “tree”, “grass/bare soil”, “built”, and “paved” classes were developed using polygons digitized from visual interpretation of the 0.6 m QuickBird panchromatic band. The ANN algorithm produced a land cover classification with an overall accuracy of 80% and Kappa of 0.74, which was more accurate than those produced by other classification algorithms evaluated, such as maximum likelihood. The “built” class had 24% errors of omission and 20% errors of commission, while the “tree” class had 7% errors of omission and 10% errors of commission. Most of the Greater Puntarenas area is limited by natural barriers including open water and mangroves, so changes in land cover classes caused by urbanization from 2002 to 2003 were assumed to be minimal.
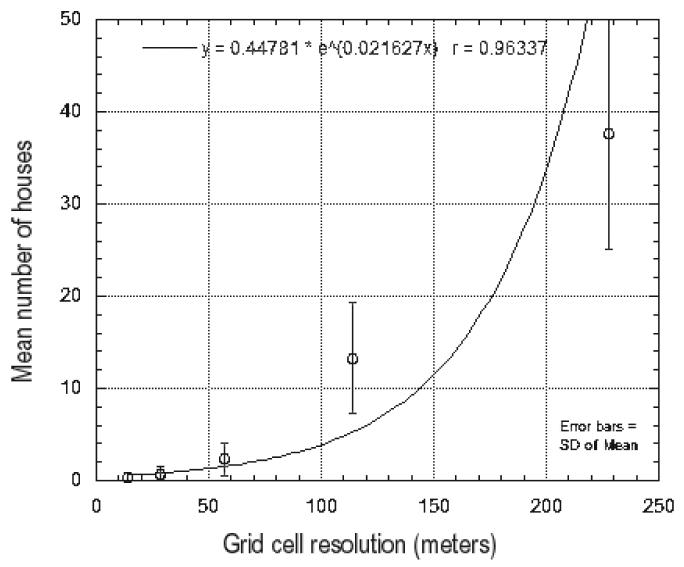
The “built” class from the land cover map provided patches of pixels that represented individual houses and small buildings of Puntarenas. Since ASTER imagery was already available, grids of different sizes were obtained from it and were used to estimate the mean number of houses/small buildings per cell extracted from the land cover map. According to the mean number of houses/small buildings per area, an optimal grid cell area that would be operationally adequate was estimated at 10 000 m2 (Figure 1). At this cell size, the number of houses per cell was approximately normally distributed and contained 13±6 houses (Shapiro-Wilk normality test W=0.976, p=0.738). A smaller cell size would not contain enough houses and would require traveling long distances frequently. Therefore, a cell size of 100 by 100 m was considered large enough for a team of two people to search in half a day (approximately three h at 15 min per house). A final grid was created using the multispectral Quickbird imagery, and cells were grouped according to each of the ten localities of Puntarenas included in the cover map. This final grid contained cells 42 by 42 pixels (100.8 × 100.8 m), and only the 355 cells that had more than 90% of their area within one specific locality of Puntarenas were included in the sampling frame. This would allow a stratified sampling method (below) and guarantee that every larval habitat found in a grid cell searched could be considered as belonging to one locality.
Figure 1.
Grid cell sizes from ASTER and mean house numbers estimated from QuickBird for Puntarenas, Costa Rica.
Cells were numbered and a stratified random sample was selected from each locality, which was proportional to the total number of cells. Localities in Puntarenas have been geographically determined by the Ministry of Health, and they correspond to proximate groups of houses, where people usually share socioeconomic characteristics, to be serviced by a local health clinic. The stratified sampling method was performed to ensure at least one sample set of each of the ten localities, which would improve representativeness of the total sample. The random sample consisted of 36 cells, approximately 10% of the total 355 cells (Figure 2). This number of grid cells selected was such that the time taken to collect the field data would not exceed three weeks, since in this case it would be necessary for the entomological data to be analyzed within approximately homogeneous external environmental conditions of each season.
Figure 2.
Sampling frame developed for the Greater Puntarenas area, Costa Rica, showing the random sample of cells (10%) selected for the entomological field studies.
To initially assess the representativeness of the selected sample grid cells, the QuickBird land cover map was used to extract the proportion of tree area (“tree” class Kappa = 0.91) in individual grid cells, as well as in the total area of the localities. Tree cover was evaluated because larval habitats have been associated to shade and specially vegetation (Barrera et al. 2006, Vezzani et al. 2005). For each locality, the mean percentage of tree cover in the selected sample cells was compared to the mean percentage of tree cover in the total cells and the percentage of tree cover in the total area of the locality.
The resulting grid with the selected cells was overlaid on the QuickBird panchromatic image for identification and visualization of the location and limits of the specific cells. The maximum and minimum coordinates for the selected cells also served to determine their position while the teams were in the field with global positioning system (GPS) units. By displaying the cells on the QuickBird panchromatic image, printing the images, and taking them to the field, small features that served as visual limits for the survey cells like roads, houses, and trees could be identified (Figure 3).
Figure 3.
Sampled cell from Puntarenas (100.8 by 100.8 m), displaying the detailed structures of the area to be analyzed in the background QuickBird panchromatic image (60 cm spatial resolution).
The first entomological survey that applied the sampling method was performed during the wet season 2006 (July and August). The area within each of the grid cells selected was searched for all potential larval habitats, most of which were the traditional “wet containers” (places or objects that held water for more than one day and seemed able to maintain this condition for more than 48 h). Within the grid cells, numbers were assigned to each “location”, which was any legally limited section of land that may or may not include a house or building (such as parks, streets and sidewalks, households, lots, churches, construction sites, buildings, parking lots, and schools). In the cases where the limit of the grid cell fell on the footprint of a house or building, only the structures on the north and west boundaries of the cell were considered completely (the structures on the south and east boundaries were not evaluated). This method would cancel out the additional and missing portions of the properties in the limits of the cell. When there were houses in the grid cells, the number of persons living in the house was noted.
All possible habitats were characterized according to their location (household or non-household and private or public area), type, and size. When present, all pupae and a sample of the larvae were collected and processed as had been performed in other areas of Costa Rica (Calderon-Arguedas et al. 2004a, 2004b). The specimens were transported in glass vials with 70% ethanol to the Medical Arthropodology Laboratory, University of Costa Rica, where they were cleared in lactophenol, mounted in Hoyer's medium, and identified. The presence of Ae. aegypti larvae, as well as the number of Ae. aegypti pupae was specially noted in order to determine the container index, location index, Breteau location index, pupae per area, and pupae per person. These larval indices are analogous to traditional Container, House (or Premise), and Breteau indices (Focks 2003) but consider all household and non-household locations in their calculation:
Container index: Number of habitats positive for Ae. aegypti larvae and/or pupae per 100 potential habitats.
Location index: Number of locations positive for Ae. aegypti larvae and/or pupae per 100 locations.
Breteau location index: Number of habitats positive for Ae. aegypti larvae and/or pupae per 100 locations.
RESULTS
The initial assessment of the selected sample grid cells showed representativeness in terms of tree cover for most of the localities. In eight of ten localities the difference between the estimated percentage of tree cover (from sample cells) and the real percentage of tree cover was less than 3% (Table 1). The proportions of tree cover and built area are being used for detailed analyses of urban structure and dengue, which will be published elsewhere.
Table 1.
Comparison of the average tree cover extracted from sample cells and the total cells in the localities of Puntarenas, Costa Rica.
| Locality | % tree of total area |
% tree mean of total cells |
% tree mean of sample |
Standard Error * | Difference† |
|---|---|---|---|---|---|
| Barrio El Carmen | 22.62 | 25.15 | 22.15 | 5.51 | −3.00 |
| El Centro | 15.36 | 16.35 | 17.75 | 3.84 | 1.40 |
| El Cocal | 18.82 | 19.07 | 29.74 | 5.80 | 10.67 |
| Veinte de Noviembre | 41.46 | 40.60 | 49.31 | 8.05 | 8.71 |
| Chacarita | 26.51 | 20.82 | 18.84 | 5.96 | −1.98 |
| Fray Casiano | 41.82 | 38.28 | 38.55 | 4.77 | 0.27 |
| San Luis | 50.53 | 48.09 | 47.85 | 9.54 | −0.24 |
| Carrizal | 40.37 | 41.38 | 42.48 | 9.58 | 1.10 |
| El Huerto | 54.82 | 54.38 | 56.75 | 19.61 | 2.37 |
| Linda Vista | 54.36 | 50.21 | 48.47 | 12.46 | −1.74 |
| Total area | 33.60 | 32.97 | 35.08 | 3.20 | 2.11 |
Standard error corrected for finite populations: [population standard deviation / n½] [(total cells-n)/(total cells-1)]½
Difference = % tree mean sample − % tree mean total cells.
During the wet season survey, a total of 581 locations were searched for mosquito larval habitats. The locations included mostly houses, but also many non-household locations such as empty lots, streets, parks, soccer fields, public schools, churches, offices, and commercial structures (Table 2). Twenty-six locations that were not categorized as houses harbored one or more positive containers, which represent 26.3% of all larvae-positive locations. Of 830 potential habitats observed, 20.6% were found in non-household locations (9.3% were in public areas), and 16.7% had mosquito larvae and/or pupae. Of mosquito-positive habitats, 29.5% were not in or around houses, and most of these habitats were observed in empty private lots. Most of the positive habitats (78%) contained immature stages of Ae. aegypti, and the second most abundant species was Culex quinquefasciatus (in 15.8% of positive containers). If only the houses were searched for mosquito larval habitats, 41 positive habitats would have been overlooked (25 containing larvae and/or pupae of Ae. aegypti and a total 85 Ae. aegypti pupae). Table 2 also presents the entomological indices when the total area in the cells is considered as opposed to only the houses. Results for the complete entomological and house surveys by locality, container profiles for wet and dry seasons, mosquito diversity, and associations with urban structure in the Greater Puntarenas area will be published elsewhere.
Table 2.
Entomological observations for households, non-household locations, and total area of the sample cells surveyed in Puntarenas, Costa Rica.
| Non-household locations (%) | |||||
|---|---|---|---|---|---|
| Households (%) | Private | Public | Total | Total (%)* | |
| No. locations evaluated | 463 (79.7) | 80 (13.8) | 38 (6.5) | 118 (20.3) | 581 (100) |
| No. locations with larvae-positive habitats |
73 (73.7) | 16 (16.2) | 10 (10.1) | 26 (26.3) | 99 (100) |
| No. potential larval habitats | 659 (79.4) | 94 (11.3) | 77 (9.3) | 171 (20.6) | 830 (100) |
| No. larvae-positive habitats | 98 (70.5) | 28 (20.1) | 13 (9.4) | 41 (29.5) | 139 (100) |
| No. Ae. aegypti pupae | 445 (84.0) | 36 (6.8) | 49 (9.2) | 85 (16.0) | 530 (100) |
| Ae. aegypti pupae per person | 0.3 | - | - | - | 0.4 |
| Container Index | 12.7 | - | - | 14.6 | 13.1 |
| Location Index | 14.0 | - | - | 14.4 | 14.1 |
| Breteau Location Index | 18.1 | - | - | 21.1 | 18.8 |
Households + total non-household locations.
DISCUSSION
These results support the geographical sampling strategy reported and show that it would yield slightly different and more exact entomological indices than a traditional household survey performed in the same areas. This research shows that in the urban ecosystem of Puntarenas, an important portion of the habitats containing mosquito larvae were not in or around houses. As has been reported previously (Morrison et al. 2006, Mahadev et al. 2004), these locations should be considered when studying mosquito ecology or diversity in urban areas, as well as for directing source reduction activities in dengue prevention and control. As in all studies, the main objective and resources available will determine the best sampling method, although calculating the entomological indices “geographically” would be more accurate than using only household surveys for entomological surveillance in most cases. There would be more detailed information available to direct control strategies, by providing, for example, key mosquito habitats in public areas that are not the direct responsibility of the household owners and may need to be eliminated by public health officials or the local municipality.
In spite of the cited advantages of this method during research and in areas where recent data is not available, it may not be the most suitable for continuous entomological surveillance. This method required GIS knowledge and high-resolution satellite imagery to determine the optimal cell size using the built structures per area and to accurately detect cell limits in the field. The optimal methodology would include imagery that is temporarily accurate, and this depends on how fast the urban landscape is changing in the study site. Recent satellite imagery can be costly, especially for programs in developing countries (QuickBird imagery cost is approximately USD $1,300 for the minimum area of 12 km2). Multispectral data from medium resolution sensors like Landsat and ASTER may provide an alternative that is less expensive, but their resolution does not allow for identification of individual houses, small roads, and buildings. While both ASTER and QuickBird data were used in this study, QuickBird imagery may be used to create the grids and calculate houses per area when coarser resolution imagery is not available. In some cases, aerial photography or local vector layers are available at the house level, and these can be substituted for the satellite image layer, depending on the final objective of the research and surveys. However, if an urban area is not under constant change and once the optimal cell size has been determined, imagery may be obtained every two or three years, and this geographical method may be considered more useful for confirmation and quality control than constant surveillance.
In this report, remote sensing and GIS technology provided useful tools to develop a sampling frame for field studies within urban Puntarenas. Although a common approach in entomological studies, sampling areas known to have high mosquito densities may result in significant selection bias. The sampling methodology applied in Puntarenas builds on the strategy proposed for sampling malaria vectors (Keating et al. 2003), which used coarser resolution satellite imagery. However, the method presented in this report shows that detail provided by high-resolution satellite imagery allows more precise calculations of optimal cell size, as well as useful information for pinpointing specific locations in urban areas and planning operations previous to the site visit. Although high-resolution satellite imagery and GIS were used to evaluate urban areas and randomly select sections aimed at obtaining data on mosquito larval habitats, this method could be applied to sample other interactions and disease systems in urban and peri-urban environments such as malaria, lymphatic filariasis, Chagas disease, and leishmaniasis. Although no entomological data was available in Puntarenas, it is possible that the selection of the cells and cell size would vary if information on vector densities, larval indices, and disease incidence is available, even though the main geographical method and principle will still be applicable. These strategies would reduce bias and provide information from the field that is both practical to obtain and representative. By selecting a geographical approach to sampling in urban environments that guarantees inclusion of all vector habitats, significant improvements could be made to strategies for prevention and control of vector-borne diseases.
Acknowledgments
We thank Joseph Keating for his comments on the manuscript; Mayra E. Solano and Adrián Avendaño for their helpful suggestions and efforts during the sampling and field surveys; and Lissette Retana, Nelson Mena, Iván Coronado, and Christian Fonseca for their extensive efforts in performing the field surveys.
This research was supported by Grant Number P20 RR020770 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. AT and OC were also supported by the University of Costa Rica and project VI-803-A6-401, and JCB by the Abess Center for Ecosystem Science and Policy, University of Miami.
REFERENCES CITED
- Barrera R, Amador M, Clark GC. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am. J. Trop. Med. Hyg. 2006;74:290–302. [PubMed] [Google Scholar]
- Beck LR, Lobitz BM, Wood BL. Remote sensing and human health: new sensors and new opportunities. Emerg. Infect. Dis. 2000;6:217–226. doi: 10.3201/eid0603.000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Arguedas O, Troyo A, Solano ME. Diversidad larval de mosquitos (Diptera: Culicidae) en contenedores artificiales procedentes de una comunidad urbana de San José, Costa Rica. Parasitol. Latinoam. 2004a;59:132–136. [Google Scholar]
- Calderón-Arguedas O, Troyo A, Solano ME. Caracterizacion de los sitios de multiplicacion de Aedes aegypti (Diptera: Culicidae) en el caserio “La Carpio”, San Jose, Costa Rica durante la estacion seca del año 2003. Rev. Biomed. 2004b;15:73–79. [Google Scholar]
- Chadee DD. Surveillance for the dengue vector Aedes aegypti in Tobago, West Indies. J. Am. Mosq. Contr. Assoc. 2003;19:199–205. [PubMed] [Google Scholar]
- Chadee DD. Key premises, a guide to Aedes aegypti (Diptera: Culicidae) surveillance and control. B. Entomol. Res. 2004;94:201–207. doi: 10.1079/ber2004297. [DOI] [PubMed] [Google Scholar]
- Focks DA, Chadee DD. Pupal survey: An epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am. J. Trop. Med. Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- Focks DA. A review of entomological sampling methods and indicators for dengue vectors. World Health Organization; Geneva: 2003. Document WHO/TDR/IDE/Den/03.1. [Google Scholar]
- Hay SI, Packer MJ, Rogers DJ. The impact of remote sensing on the study and control of invertebrate intermediate hosts and vectors for disease. Int. J. Remote Sensing. 1997;18:2899–2930. [Google Scholar]
- Hay SI, Omumbo JA, Craig MH, Snow RW. Earth observation, geographic information systems and Plasmodium falciparum malaria in sub-Saharan Africa. Adv. Parasitol. 2000;47:173–215. doi: 10.1016/s0065-308x(00)47009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating J, Macintyre K, Mbogo C, Githeko A, Regens JL, Swalm C, Ndenga B, Steinberg LJ, Kibe L, Githure JI, Beier JC. A geographic sampling strategy for studying relationships between human activity and malaria vectors in urban Africa. Am. J. Trop. Med. Hyg. 2003;68:357–365. [PubMed] [Google Scholar]
- Mahadev PVM, Fulmali PV, Mishra AC. A preliminary study of multilevel geographic distribution and prevalence of Aedes aegypti (Diptera: Culicidae) in the state of Goa, India. Indian J. Med. Res. 2004;120:173–182. [PubMed] [Google Scholar]
- Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, Watts D, Stancil JD, Olson JG, Blair P, Scott TW. Temporal and geographical patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos. Peru. J. Med. Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, Vidal-Ore C, Scott TW. Aedes aegypti (Diptera:Culicidae) production from non-residential sites in the Amazonian city of Iquitos. Peru. Ann. Trop. Med. Parasitol. 2006;100(Suppl 1):73–86. doi: 10.1179/136485906X105534. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. Studying the global distribution of infectious diseases using GIS and RS. Nature Rev. Microbiol. 2003;1:231–237. doi: 10.1038/nrmicro776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Wilson AJ, Hay SI, Graham AJ. The global distribution of yellow fever and dengue. Adv. Parasitol. 2006;62:181–220. doi: 10.1016/S0065-308X(05)62006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani D, Rubio A, Velazquez SM, Schweigmann N, Wiegand T. Detailed assessment of microhabitat suitability for Aedes aegypti (Diptera: Culicidae) in Buenos Aires, Argentina. Acta Trop. 2005;95:123–131. doi: 10.1016/j.actatropica.2005.03.010. [DOI] [PubMed] [Google Scholar]
- World Health Organization Outbreak of classic dengue, Costa Rica. Wkly. Epidemiol. Rec. 1994;69:85–86. [PubMed] [Google Scholar]