Abstract
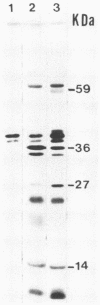
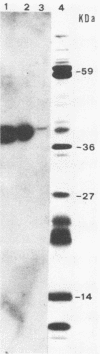
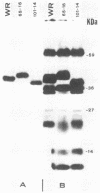
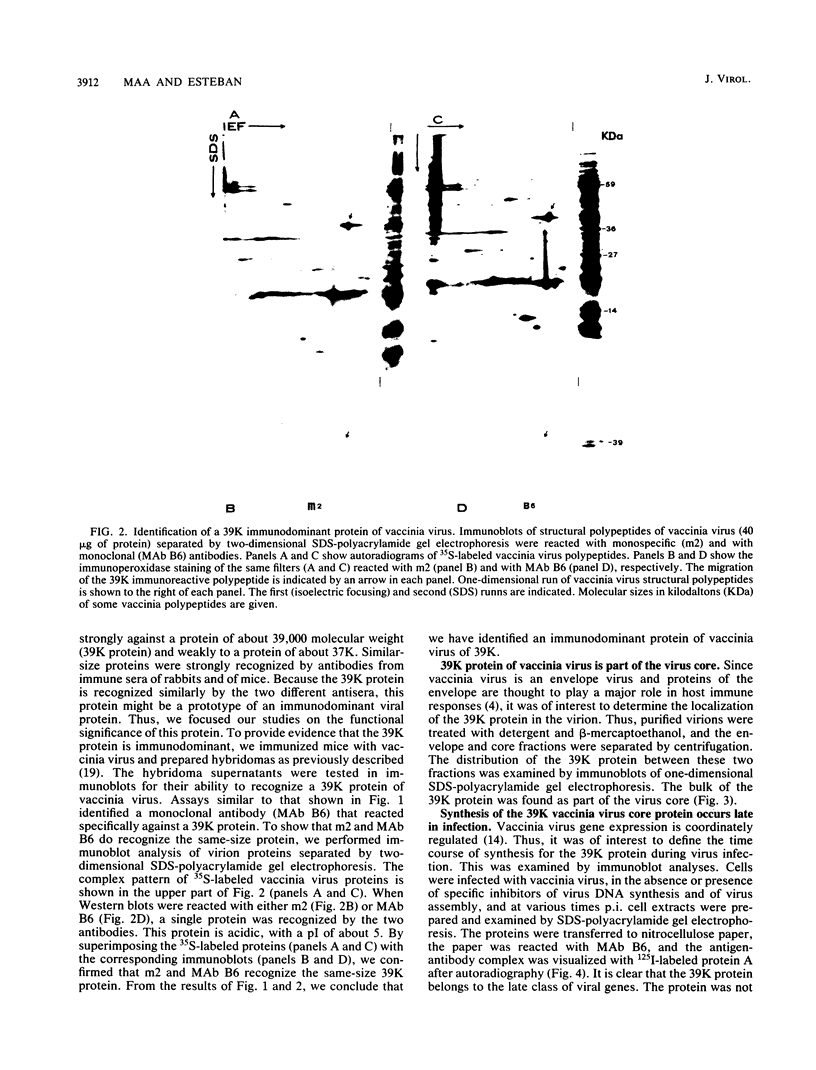
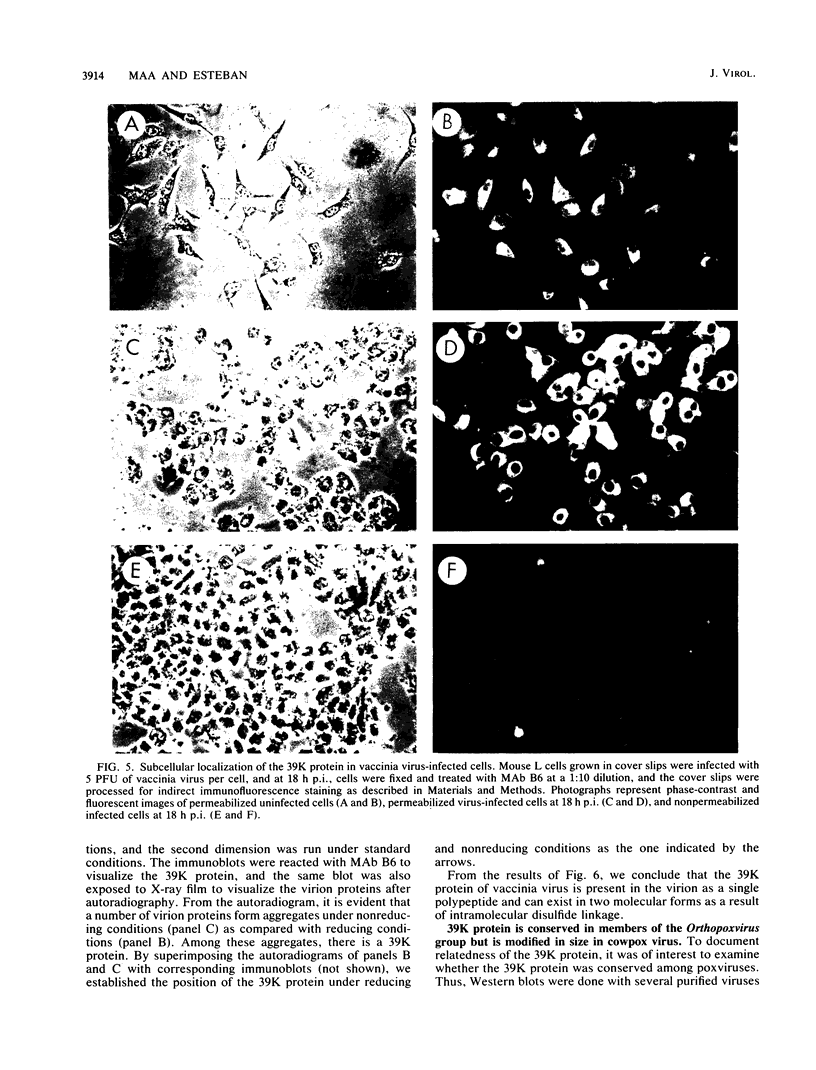
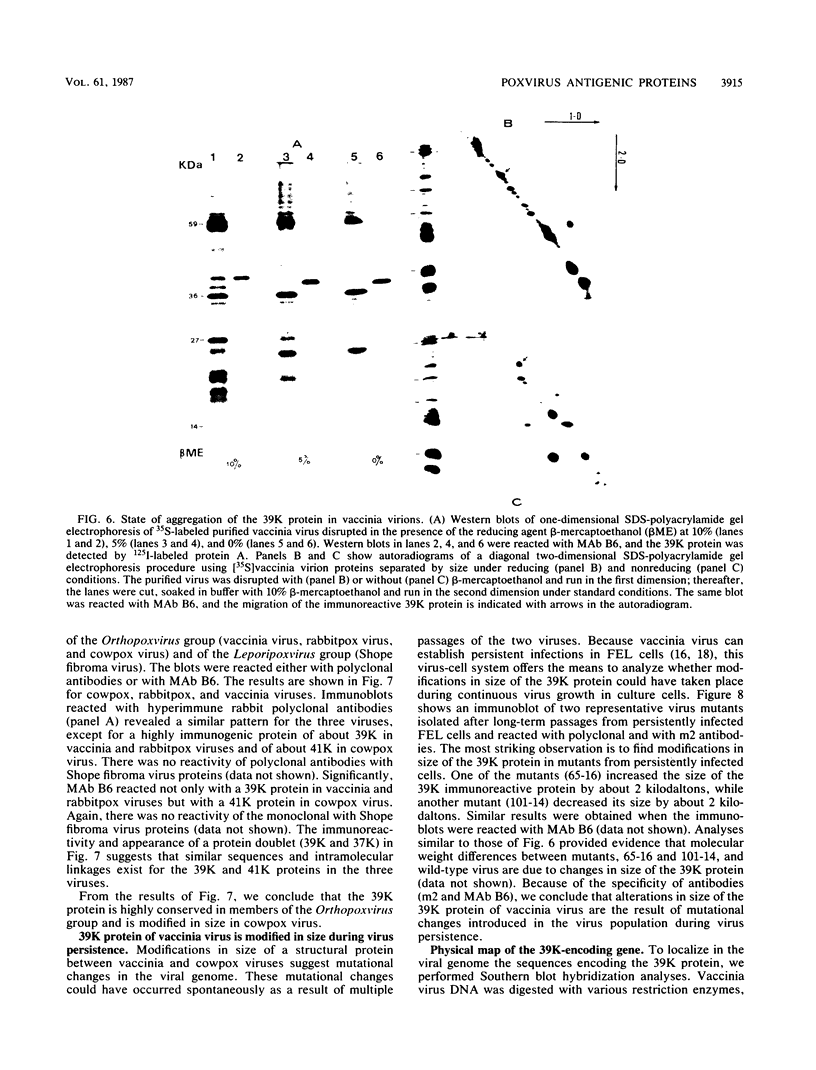
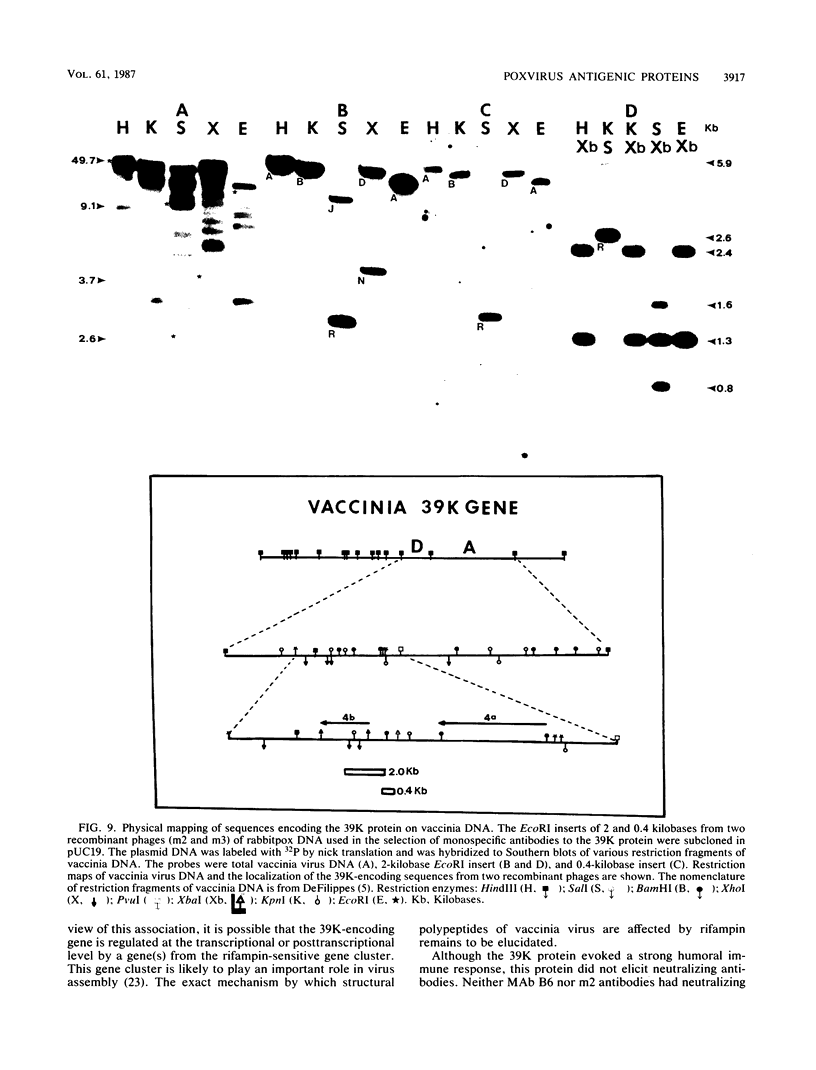
Little is known about the nature of poxvirus proteins involved in the host immune response. Screening a lambda gt11 expression library of genomic rabbit poxvirus DNA with hyperimmune rabbit anti-vaccinia virus serum and selection of monospecific antibodies identified a highly antigenic viral protein of about 39,000 molecular weight (39K protein). The same-size protein of vaccinia virus was also identified with a monoclonal antibody (MAb B6) obtained from hybridomas generated after fusion of hyperimmunized mouse spleen cells with mouse myeloma cells. Structural analysis revealed that the 39K protein is an acidic polypeptide, that it can exist in two molecular forms because of intramolecular disulfide linkages, and that it is part of the virus core. This protein shares antigenic determinants with a cytoplasmic component(s) from uninfected cells. Functional studies revealed that the 39K protein is synthesized at late times postinfection and appears to be required for virus assembly. This protein is highly conserved in members of the Orthopoxvirus group, but in cowpox virus, a 41K virion protein was specifically recognized by antibodies that reacted against the vaccinia virus 39K protein. Significantly, during long-term passages of Friend erythroleukemia cells persistently infected with vaccinia virus, some virus mutants were found to increase or decrease by about 2 kilodaltons the size of the 39K protein. Mapping analysis localized sequences encoding the 39K protein in a rifampin-sensitive gene cluster between the two major core-associated viral polypeptides, 4a and 4b. The fact that the 39K core protein of vaccinia virus elicits strong humoral immune response, induces antibodies that react against a host component(s), and is subjected to genetic variability suggests that this protein has important biological functions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behbehani A. M. The smallpox story: life and death of an old disease. Microbiol Rev. 1983 Dec;47(4):455–509. doi: 10.1128/mr.47.4.455-509.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Bravo R. Specific proteins synthesized during the viral lytic cycle in vaccinia virus-infected HeLa cells: analysis by high-resolution, two-dimensional gel electrophoresis. J Virol. 1986 May;58(2):569–577. doi: 10.1128/jvi.58.2.569-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Fujinami R. S., Oldstone M. B. Infection with vaccinia favors the selection of hybridomas synthesizing autoantibodies against intermediate filaments, one of them cross-reacting with the virus hemagglutinin. J Immunol. 1983 Sep;131(3):1546–1553. [PubMed] [Google Scholar]
- DeFilippes F. M. Restriction enzyme mapping of vaccinia virus DNA. J Virol. 1982 Jul;43(1):136–149. doi: 10.1128/jvi.43.1.136-149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essani K., Dales S. Biogenesis of vaccinia: evidence for more than 100 polypeptides in the virion. Virology. 1979 Jun;95(2):385–394. doi: 10.1016/0042-6822(79)90493-8. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Oie M. Proteolytic activation of vaccinia virus for the penetration phase of infection. Virology. 1982 Jan 15;116(1):297–305. doi: 10.1016/0042-6822(82)90421-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- Mallon V. R., Domber E. A., Holowczak J. A. Vaccinia virus proteins on the plasma membranes of infected cells. II. Expression of viral antigens and killing of infected cells by vaccinia virus-specific cytotoxic T cells. Virology. 1985 Aug;145(1):1–23. doi: 10.1016/0042-6822(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Carter J. K., Moyer R. W. Isolation and characterization of monoclonal antibodies directed against two subunits of rabbit poxvirus-associated, DNA-directed RNA polymerase. J Virol. 1985 Sep;55(3):670–680. doi: 10.1128/jvi.55.3.670-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Katz E., Grimley P. M. Rifampicin: a specific inhibitor of vaccinia virus assembly. Nature. 1969 Dec 27;224(5226):1280–1284. doi: 10.1038/2241280a0. [DOI] [PubMed] [Google Scholar]
- Paez E., Dallo S., Esteban M. Generation of a dominant 8-MDa deletion at the left terminus of vaccinia virus DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3365–3369. doi: 10.1073/pnas.82.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Friend C. Persistent infection of Friend erythroleukemia cells with vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4805–4809. doi: 10.1073/pnas.79.15.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Janeczko R., Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985 Nov;56(2):482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Kahn J. S., Esteban M. Molecular cloning, encoding sequence, and expression of vaccinia virus nucleic acid-dependent nucleoside triphosphatase gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9566–9570. doi: 10.1073/pnas.83.24.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Paez E., Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987 Feb;61(2):395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel J., Moss B. Transcriptional and translational mapping and nucleotide sequence analysis of a vaccinia virus gene encoding the precursor of the major core polypeptide 4b. J Virol. 1985 Dec;56(3):830–838. doi: 10.1128/jvi.56.3.830-838.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J., Paoletti E. Physical mapping and DNA sequence analysis of the rifampicin resistance locus in vaccinia virus. Virology. 1985 Dec;147(2):394–404. doi: 10.1016/0042-6822(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Piccini A., Paoletti E. Vaccinia virus rifampicin-resistance locus specifies a late 63,000 Da gene product. Virology. 1986 Apr 15;150(1):45–54. doi: 10.1016/0042-6822(86)90264-3. [DOI] [PubMed] [Google Scholar]
- Weinrich S. L., Hruby D. E. Noncoordinate regulation of a vaccinia virus late gene cluster. J Virol. 1987 Mar;61(3):639–645. doi: 10.1128/jvi.61.3.639-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton S., Gordon J., Dales S. Identification of antigenic determinants by polyclonal and hybridoma antibodies induced during the course of infection by vaccinia virus. Virology. 1986 Jan 15;148(1):84–96. doi: 10.1016/0042-6822(86)90405-8. [DOI] [PubMed] [Google Scholar]
- Wittek R., Richner B., Hiller G. Mapping of the genes coding for the two major vaccinia virus core polypeptides. Nucleic Acids Res. 1984 Jun 25;12(12):4835–4848. doi: 10.1093/nar/12.12.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]