Abstract
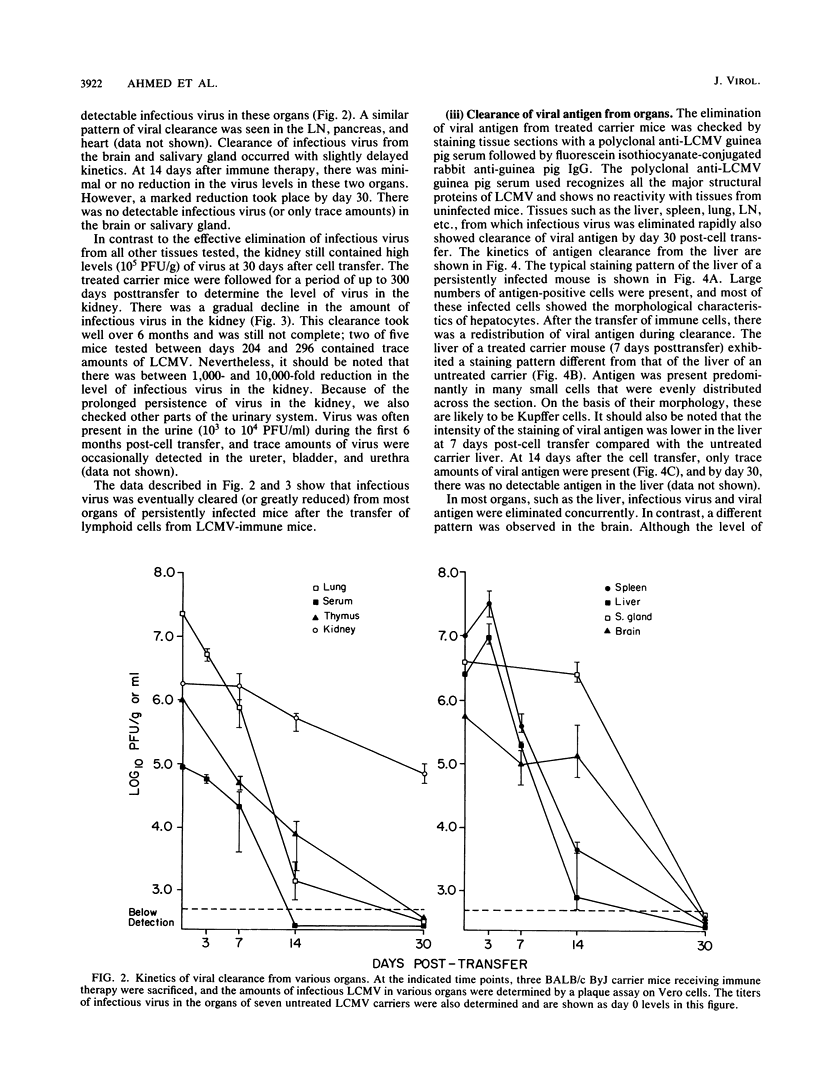
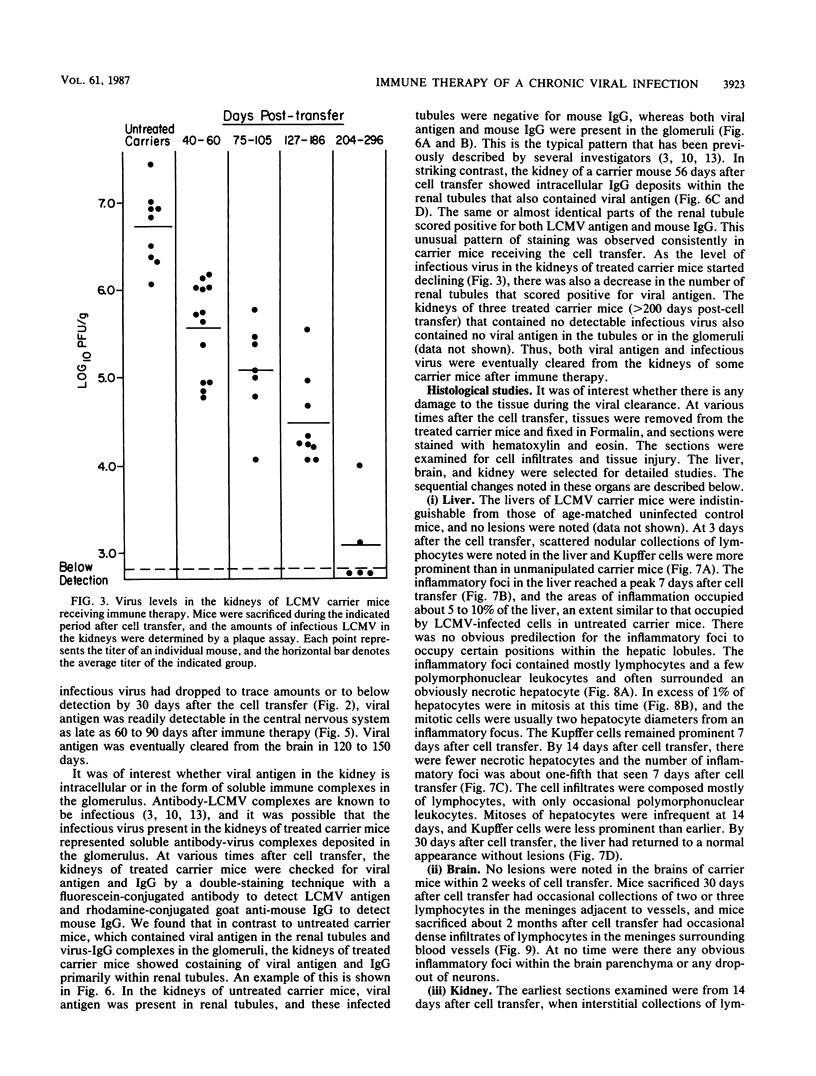
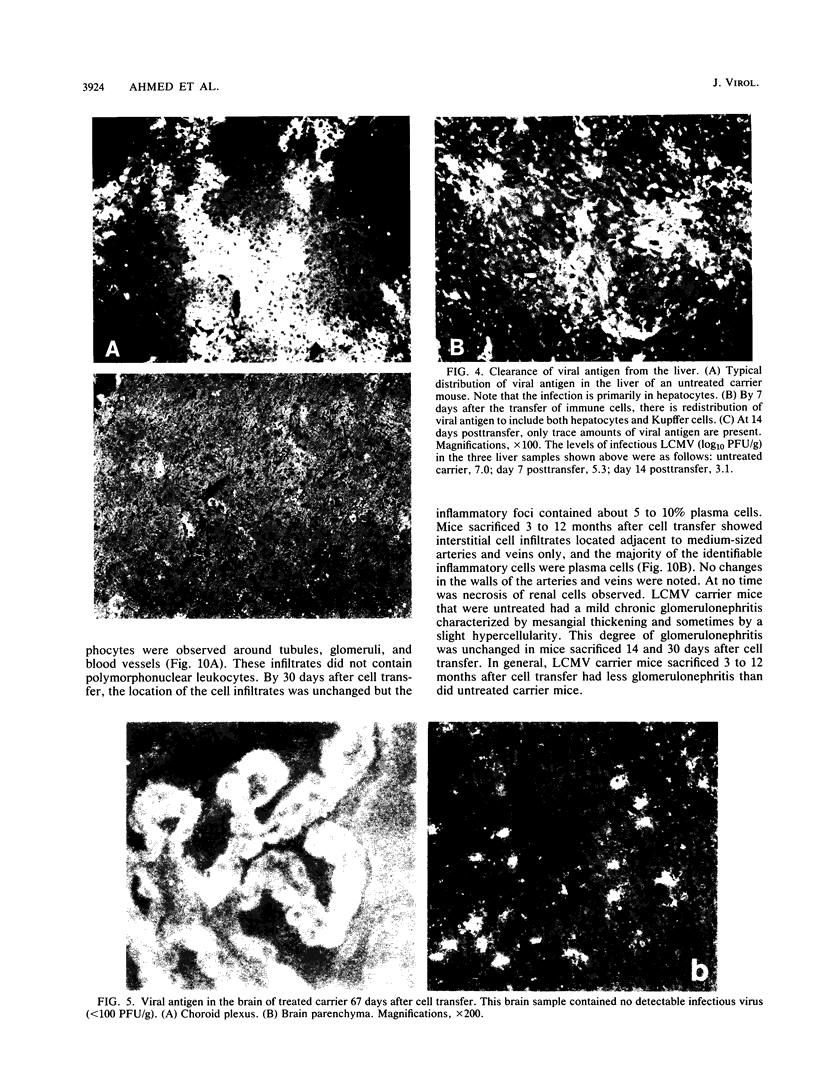
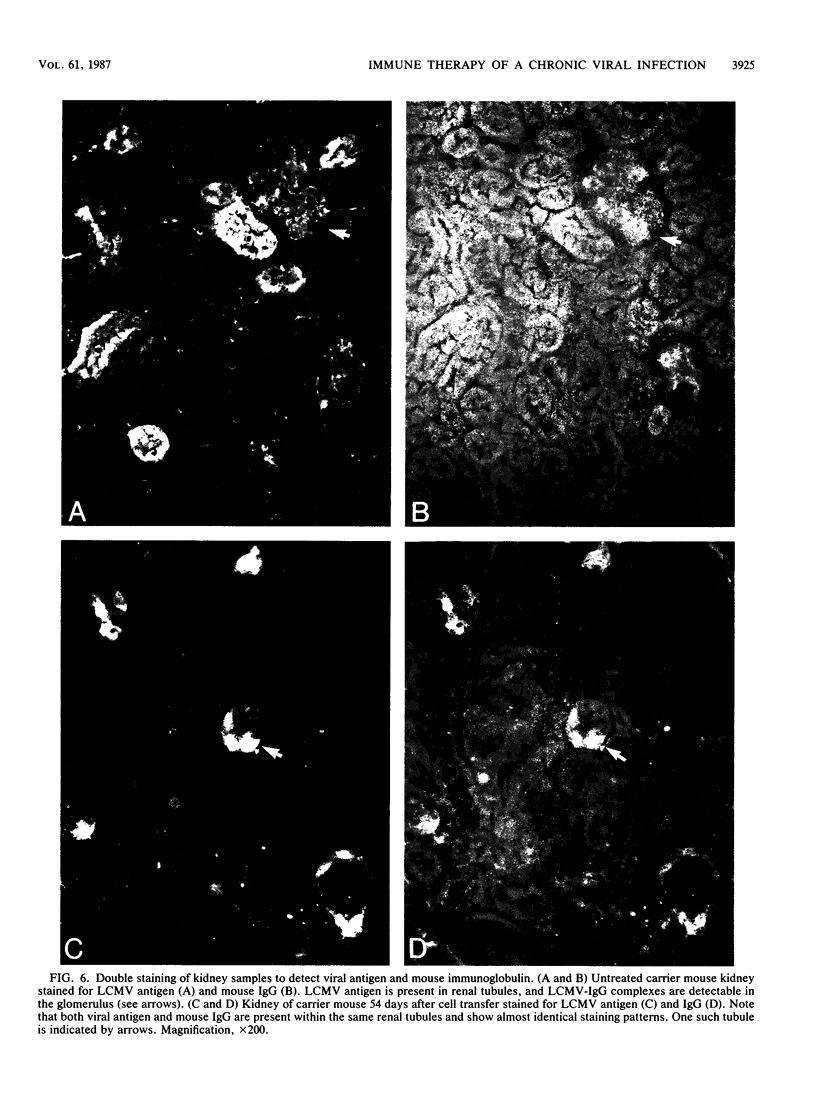
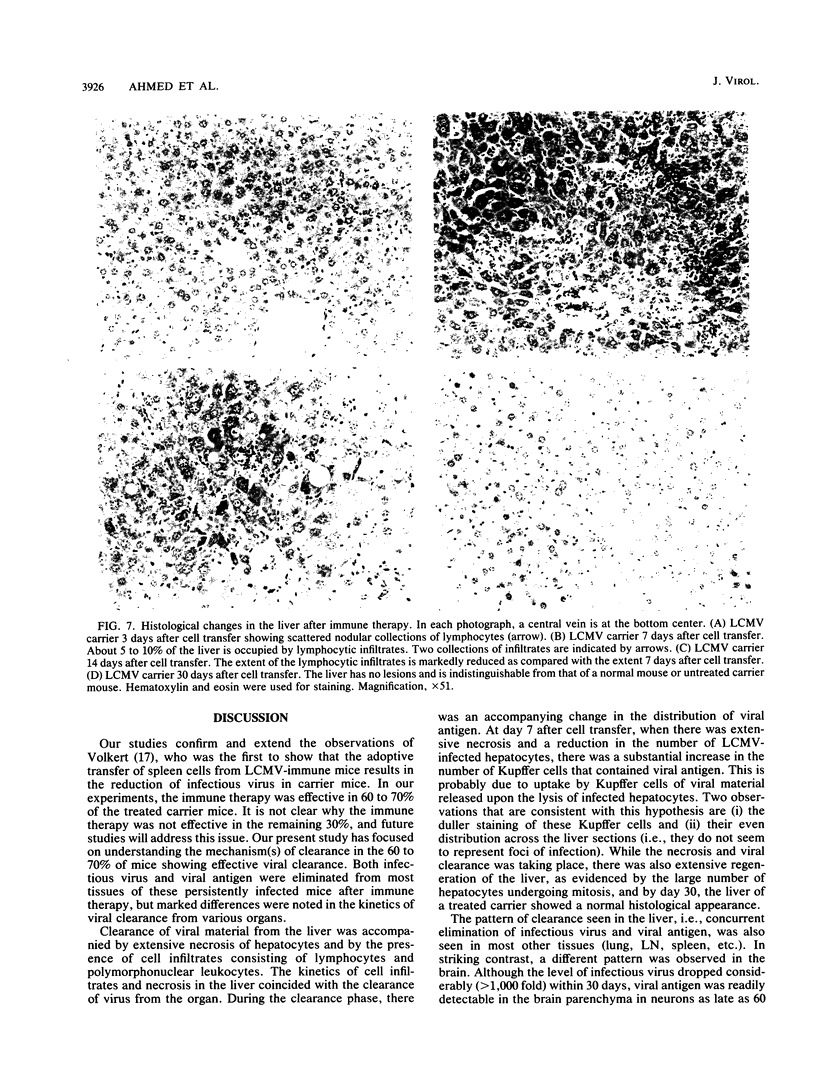
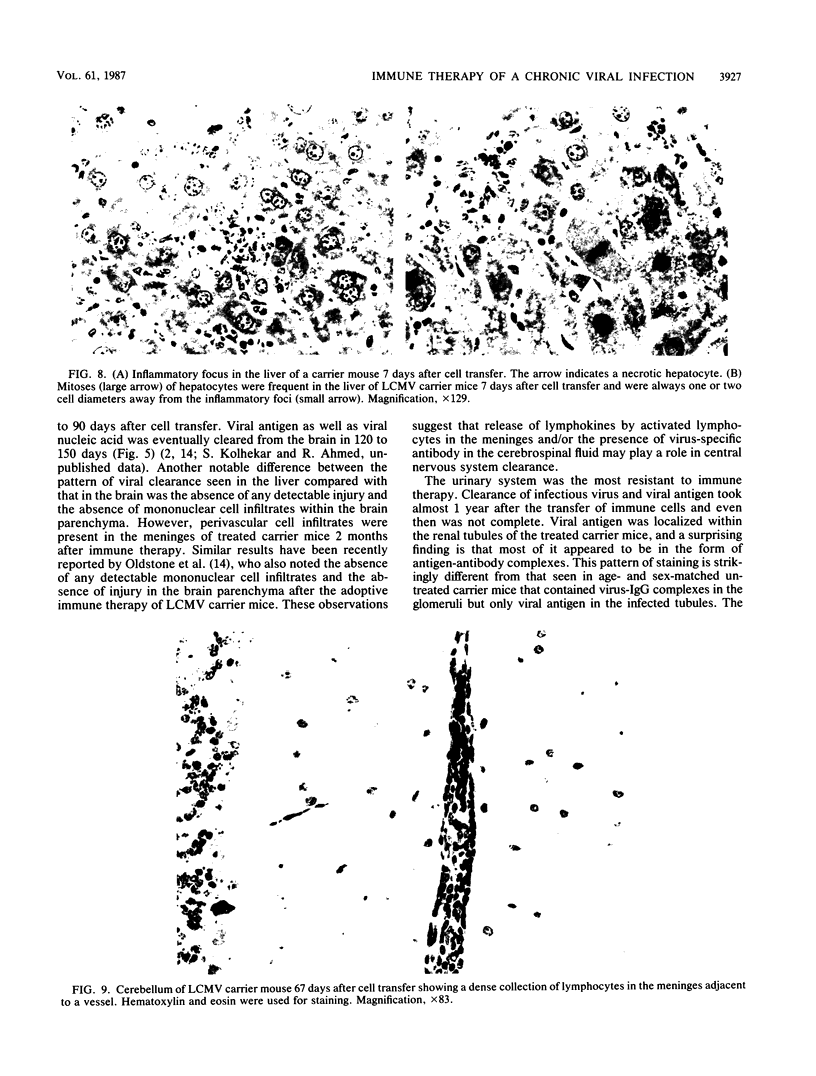
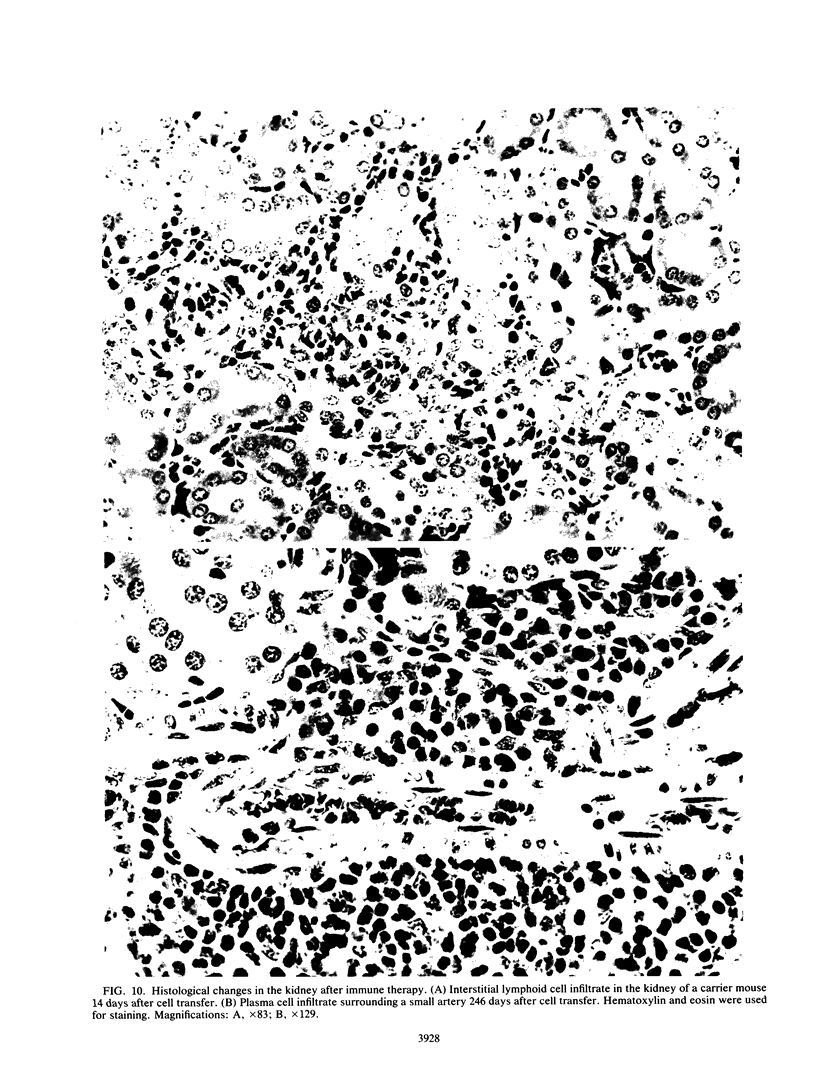
The mechanism of viral clearance was studied by using the mouse model of chronic infection with lymphocytic choriomeningitis virus. Distinct patterns of viral clearance and histopathology were observed in different organs after adoptive immune therapy of persistently infected (carrier) mice. Clearance from the liver occurred within 30 days and was accompanied by extensive mononuclear cell infiltrates and necrosis of hepatocytes. Infectious virus and viral antigen were eliminated concurrently. This pattern of viral clearance was also seen in most other tissues (i.e., lung, spleen, lymph nodes, pancreas, etc.). In contrast, a different pattern of clearance was observed in the brain. Infectious virus was eliminated within 30 days, but viral antigen persisted in the central nervous systems of treated carrier mice for up to 90 days. The urinary system was the most resistant to immune therapy. Elimination of infectious virus and viral antigen from the kidney took greater than 200 days and even then was not complete; trace levels of infectious virus were still present in the kidneys of some treated carrier mice. After immune therapy, viral antigen in the kidney was located within renal tubules that costained for intracellular mouse immunoglobulin G. This unusual staining pattern, coupled with the observation of large numbers of plasma cells within the kidney, suggests that virus-immunoglobulin G complexes found in the tubules may represent in situ immune complex formation as opposed to deposition of circulating immune complexes. In conclusion, these results suggest that the site (organ) of viral persistence is an important consideration in developing treatment strategies for controlling chronic viral infections.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Curran J. W., Morgan W. M., Hardy A. M., Jaffe H. W., Darrow W. W., Dowdle W. R. The epidemiology of AIDS: current status and future prospects. Science. 1985 Sep 27;229(4720):1352–1357. doi: 10.1126/science.2994217. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M. Patterns of persistent viral infections. N Engl J Med. 1986 Oct 9;315(15):939–948. doi: 10.1056/NEJM198610093151506. [DOI] [PubMed] [Google Scholar]
- Hoffsten P. E., Oldstone M. B., Dixon F. J. Immunopathology of adoptive immunization in mice chronically infected with lymphocytic choriomeningitis virus. Clin Immunol Immunopathol. 1977 Jan;7(1):44–52. doi: 10.1016/0090-1229(77)90028-9. [DOI] [PubMed] [Google Scholar]
- Kaysen G. A., Myers B. D., Couser W. G., Rabkin R., Felts J. M. Mechanisms and consequences of proteinuria. Lab Invest. 1986 May;54(5):479–498. [PubMed] [Google Scholar]
- Marion P. L., Robinson W. S. Hepadna viruses: hepatitis B and related viruses. Curr Top Microbiol Immunol. 1983;105:99–121. doi: 10.1007/978-3-642-69159-1_2. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Ahmed R., Byrne J., Buchmeier M. J., Riviere Y., Southern P. Virus and immune responses: lymphocytic choriomeningitis virus as a prototype model of viral pathogenesis. Br Med Bull. 1985 Jan;41(1):70–74. doi: 10.1093/oxfordjournals.bmb.a072029. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lymphocytic choriomeningitis: production of antibody by "tolerant" infected mice. Science. 1967 Dec 1;158(3805):1193–1195. doi: 10.1126/science.158.3805.1193. [DOI] [PubMed] [Google Scholar]
- Seiler M. W., Hoyer J. R. Ultrastructural studies of tubulointerstitial immune complex nephritis in rats immunized with Tamm-Horsfall protein. Lab Invest. 1981 Oct;45(4):321–327. [PubMed] [Google Scholar]
- VOLKERT M. STUDIES ON IMMUNOLOGICAL TOLERANCE TO LCM VIRUS. 2. TREATMENT OF VIRUS CARRIER MICE BY ADOPTIVE IMMUNIZATION. Acta Pathol Microbiol Scand. 1963;57:465–487. [PubMed] [Google Scholar]