Abstract
Vaccination with Plasmodium sporozoites attenuated by irradiation or genetic manipulation induces a protective immune response in rodent malaria models. Recently, vaccination with chemically attenuated P. berghei sporozoites (CAS) has also been shown to elicit sterile immunity in mice. Here we show that vaccination with CAS of P. yoelii also protects against homologous infection and that a P. berghei CAS vaccine cross protects against heterologous challenge with P. yoelii sporozoites. Vaccination with P. yoelii or P. berghei CAS induced parasite-specific antibodies and IFN-γ-producing CD8+ T cells at levels not significantly different from radiation-attenuated sporozoites. Our findings provide an initial characterization of the immune response generated by CAS vaccination and suggest that this attenuation process could be used in the production of an effective cross-protective liver stage vaccine for malaria.
Keywords: chemically attenuated sporozoites (CAS), CD8+ T cells, malaria
1. Introduction
The only experimental vaccine to confer complete protection against malaria in humans utilizes attenuated Plasmodium sporozoites. Radiation-attenuated sporozoites (RAS) have been used both in rodent models and human volunteers as vaccines to elicit protective immune responses [1, 2]. Presumably, the attenuation of irradiated sporozoites occurs due to a set of random double-strand breaks in the parasite DNA that lead to a block in liver stage development [3]. However, with this method, the issue of adequate irradiation dosage is a concern since suboptimal attenuation could result in breakthrough infections [1, 4]. As an alternative, vaccines using genetically attenuated parasites (GAS) have been generated in which genes that are essential for liver-stage development are deleted. This vaccine strategy has been validated in the rodent malaria models Plasmodium berghei and Plasmodium yoelii [5-10], although the effectiveness of GAS has not been experimentally determined in humans.
Many studies have shown that infection with attenuated sporozoites induces similar immune responses using either the irradiated or genetically attenuated models. Protection induced by vaccination with both RAS and GAS sporozoites is essentially mediated by interferon (IFN)-γ-producing CD8+ T cells [8, 11, 12]. Antibodies generated in response to attenuated parasite vaccines also contribute to protection, but CD8+ T cells are believed to play the major protective role [12, 13].
Recently, we have shown that sporozoites can also be attenuated using the DNA-binding drug, centanamycin [14]. These chemically-attenuated sporozoites (CAS) are generated by treatment of sporozoites in vitro with centanamycin and vaccination with CAS protects two mouse strains against homologous challenge with P. berghei [14]. RAS and GAS vaccines from both P. berghei and P. yoelii have been shown to induce protection in mice [2, 5-7, 10, 15, 16]. Here, we report that CAS vaccines are also protective using a homologous prime-boost schedule with P. yoelii, and that this strategy also cross-protects mice when immunized with P. berghei and challenged with P. yoelii 10 days after the final immunization. Heterologous protection was not seen, however when the challenge was delayed to 21 days. We also show that high levels of CD8+ T cells and antibodies are generated in response to immunization with CAS, suggesting that the immune effector mechanisms induced by CAS vaccination are similar to those induced by RAS and GAS vaccines.
2. Materials and Methods
2.1. Attenuation of sporozoites
Anopheles stephensii mosquitoes were maintained as described [17] and infected with P. berghei ANKA PbGFPCON [18] or P. yoelii (17XNL) as indicated. Salivary glands of mosquitoes infected with P. berghei were dissected at or about day 18 post-feeding (p.f.) and kept on ice. Plasmodium yoelii were dissected at day 14 p.f. and kept at room temperature. Sporozoites were quantified using a hemocytometer. Centanamycin (2M) was prepared in a PET (polyethylene glycol 400, ethanol, Tween 80)/glucose solution [19]. CAS were generated by treatment with 2mM centanamycin diluted in DMEM, while control sporozoites were treated with the same volume of vehicle. Sporozoites were incubated with centanamycin or vehicle for 30 min at room temperature for all immunizations, or 30, 60, or 90 min at room temperature for the membrane integrity assay, centrifuged at 21,000 × g for 7 min and resuspended in DMEM. RAS were generated by exposure of dissected sporozoites to a γ-irradiator (MDS Nordion Gammacell 1000 Elite) at a dose of 120 Gy. For each experiment, control, CAS and RAS sporozoites were always generated from the same initial pool of sporozoites.
2.2. Membrane integrity
Assessment of membrane integrity in P. yoelii 17NXL sporozoites was completed as described [14] except that after incubation, 200 sporozoites were counted per group, per experiment.
2.3. Immunization and challenge
Procedures for all animal experiments were approved by New York School of Medicine Institutional Animal Care and Use Committee. Eight-week old female BALB/c mice were initially immunized with 2 × 104 or 5 × 104 P. yoelii or 5 × 104 P. berghei RAS or CAS, as indicated, and in the case of multiple immunizations, were then boosted two additional times at 7 to 9 day intervals with 2 × 104 P.yoelii or P. berghei RAS or CAS, as indicated. Challenges were completed with 100 P. yoelii untreated sporozoites 10 or 21 days after the final boost, as indicated. At each immunization and challenge, 2 to 5 age-matched, naïve mice were injected with control sporozoites from the same mosquito batch as the experimental groups. Parasitemia was evaluated from day 2 p.i. onwards by Giemsa-stained thin blood smears. Protected mice were followed for at least 28 days, while control mice were followed until the parasitemia peaked (at about 30% for P. yoelii or 80% for P. berghei) and were euthanized.
2.4 Enzyme-linked immunospot assay (ELISPOT)
An additional experiment was completed using the P. yoelii and P. berghei multiple immunization strategy. Ten days after the final boost, the mice immunized with CAS and RAS were sacrificed and the spleen mononuclear cells were isolated by disruption in a 100μm cell strainer using the end of a sterile 3 mL syringe plunger. Age-matched naïve control mice and mice that received control sporozoites were also included. Cells were washed and resuspended in ACK (ammonium-chloride-potassium) lysing buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.3). The spleen cells were then counted on a hemocytometer and 2-fold dilutions of the cells were plated in triplicate. A20 cells (ATCC TIB-208) were used as antigen-presenting cells. The cells were coated with a H-2Kd-specific CD8+ peptide from the P. yoelii CSP (SYVPSAEQI), or the P. berghei CSP (SYIPSAEKI) and incubated with splenocytes for 48 h. Epitope-specific CD8+ T cells were quantified using an IFN-γELISPOT assay as described [20]. Purified anti-mouse IFN-γ (R4) and biotinylated anti-mouse IFN-γ (XMG1.2) were obtained from BD Pharmingen. Plates were processed and dried at room temperature, and the spot forming cells were counted on a CTL ImmunoSpot Plate Reader (Series 3) using default settings. Assays were performed in triplicate for each dilution of spleen cells from each mouse using antigen-presenting cells that were either unprimed (to show background) or primed with the peptide.
2.5 Immunofluorescence titration
For titration of P. yoelii- and P. berghei-specific antibody levels, salivary-gland sporozoites were air-dried on glass Multiwell IFA slides. Mouse serum from the same animals as processed for the ELISPOT assay was titrated and primary antibody bound to sporozoites was detected using FITC-labeled anti-mouse IgG. A monoclonal CSP-specific antibody (2F6) was used as a positive control. Each titration was completed in triplicate for each dilution and each mouse.
2.6 Statistical analysis
Statistical analysis was completed using Prism (v. 4.0a). Normality was tested in the ELISPOT assay using the Kolmogorov-Smirnov Goodness-of-Fit Test. Data with a p value > 0.10 were considered normal. The differences were then tested using an ANOVA with a Tukey's Multiple Comparison post-hoc test. Significant differences between groups are indicated. Fisher exact test was used to calculate p values in protection experiments (Table 1).
Table 1.
Protection of mice immunized with CAS or RAS against challenge with wild-type sporozoites
| Group | Mouse strain | Immunizationa RAS/CAS × 103 | Challengeb | Day of challenge | Sterile immunity? | Prepatent period (days) | No. protected (no. challenged) | ||
|---|---|---|---|---|---|---|---|---|---|
| Controlc |
CASd |
RAS |
|||||||
| 1 | BALB/c | 50/20/20 (P.y.) | 100 (P.y.) | 10 | yes | NAe | 0 (8) | 4 (4) | 4 (4) |
| 2 | BALB/c | 20 (P.y.) | 100 (P.y.) | 21 | no | 1.5f | 0 (5) | 0 (5) | 0 (5) |
| 3 | BALB/c | 50/20/20 (P.b.) | 100 (P.y.) | 10 | yes | NA | 0 (8) | 4 (4) | 4 (4) |
| 4 | BALB/c | 50/20/20 (P.b.) | 100 (P.y.) | 21 | no | <2g | 0 (11) | 0 (8) | 0 (8) |
Groups of mice were immunized with P. berghei ANKA (P.b.) or P. yoelii 17XNL (P.y.) control (vehicle-treated) sporozoites, CAS, or RAS as indicated, isolated from the same mosquito batches and were immunized at 7 day intervals.
Groups of mice were challenged with P. berghei ANKA or P. yoelii 17XNL wild-type sporozoites
Naïve, age-matched mice were used at the time of all immunizations and challenges.
Statistical analysis using Fisher exact test for comparison of Control and CAS groups gave p<0.002 for groups 1 and 3.
NA, not applicable.
Naïve, age-matched mice developed patent parasitemia 1.5 days p.i. in both the CAS and RAS groups.
All mice showed patent parasitemia 2 days p.i.
3. Results
3.1 Immunization of mice with CAS protects against homologous and heterologous challenge
We evaluated the ability of CAS of P. yoelii to produce sterile protection in the mouse malaria model. First, we tested the membrane integrity of sporozoites treated with centanamycin using propidium iodide uptake experiments to ensure that centanamycin treatment did not result in decreased viability of sporozoites relative to controls, as observed with centanamycin-treated P. berghei sporozoites [14]. Similar low level labeling with propidium iodide was observed in control and centanamycin-treated sporozoites, suggesting that centanamycin did not affect membrane integrity (Fig. 1). A higher percentage of P. yoelii 17XNL sporozoites remained viable after 90 minutes at room temperature (94.8 % ± 1.77 SD in the control group and 96.8 % ± 1.77 SD in the centanamycin-treated group; Fig 1) compared with the viability of P. berghei ANKA sporozoites (65.5 % ± 0.95 SD in the vehicle-treated group and 62.0 % ± 1.42 SD in the centanamycin-treated group) as described previously [14].
Figure 1. Treatment of P. yoelii 17 XNL sporozoites in vitro does not affect sporozoite membrane integrity.
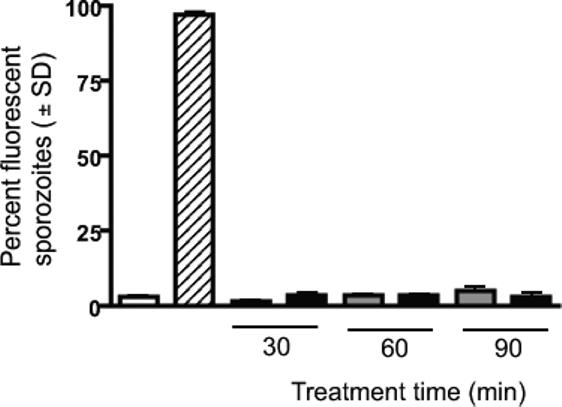
Sporozoites were incubated with vehicle (gray bars) or 2mM centanamycin (black bars) for 30, 60, or 90 min before addition of propidium iodide. Control sporozoites were either tested immediately following dissection (open bar) or heat killed (striped bar) for 15 min at 65°C before counting. For each sample, 200 sporozoites were counted in two separate experiments, and the average percentage of staining with propidium iodide is shown.
Since P. yoelii sporozoites were viable after treatment with centanamycin, they were evaluated as a homologous CAS vaccine. Groups of mice (BALB/c) were given an initial immunization dose of 5 × 104 CAS or RAS (as a positive control), and two subsequent doses of 2 × 104 CAS or RAS, for a total of 3 immunizing doses, at 7 day intervals (Group 1, Table 1). Mice were challenged with 100 untreated, wild-type P. yoelii sporozoites 10 days after the final immunizing dose. All mice that received either the RAS or CAS were fully protected against homologous P yoelii challenge (Group 1, Table 1). Immunization with one dose of either CAS or RAS was not protective with homologous P. yoelii challenge (Group 2, Table 1), but resulted in a 1.5-day delay in patent parasitemia for both CAS- and RAS-immunized mice. To evaluate the ability of a heterologous CAS vaccine to induce cross protection against challenge with different parasite species, mice were immunized with P. berghei CAS (or RAS as a positive control) using the same schedule, followed by heterologous challenge with P. yoelii sporozoites: this protocol also resulted in complete protection in all animals (Group 3, Table 1) only when challenge was completed 10 days after the final immunization. If the challenge was delayed to 21 days, neither RAS- or CAS-immunized mice were protected (Group 4, Table 1).
3.2 Immunization of mice with CAS induces antigen-specific IFN-γ producing CD8+ T cells
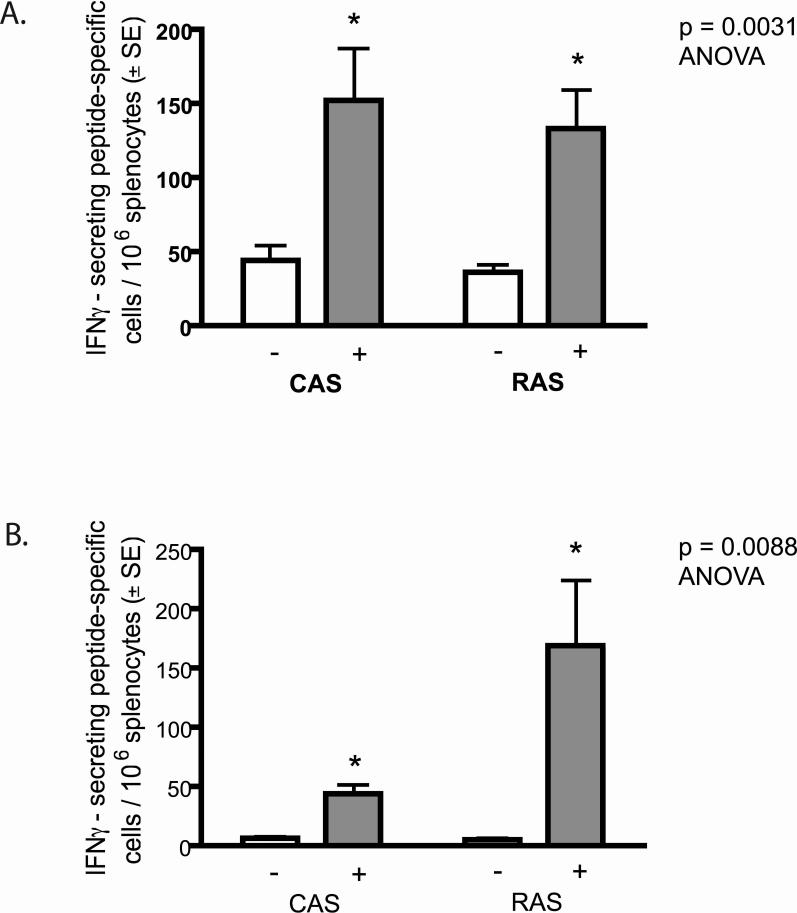
Since RAS and GAS vaccines induce antigen-specific IFN-γ producing CD8+ T cells, which mediate protection against sporozoite challenge [8, 11, 12] we evaluated the ability of a P. yoelii and P. berghei CAS vaccine to induce CD8+ T cell responses to a species-specific CSP epitope. We immunized BALB/c mice with three doses (5 × 104, 2 × 104, and 2 × 104 at 7-day intervals) of CAS and RAS (as a positive control). Ten days after the final immunization, we measured IFN-γ secretion by splenic T cells specific for a H-2Kd-specific CD8+ peptide from the P. yoelii CSP (SYVPSAEQI) [21] or P. berghei CSP (SYIPSAEKI) [22] using an ELISPOT. Parasite-induced IFN-γ secretion by CD8+ T cell responses in both P. yoelii and P. berghei CAS and RAS were significantly higher (p=0.0031, ANOVA, n=5 and p=0.0088, ANOVA, n=3) than background responses in unstimulated control cells (Fig. 2A and B) and splenic cells from naïve mice (data not shown). Interestingly, there was no significant difference between the number of spot forming cells in P. yoelii or P. berghei CAS (152.6 ± 34.8 SEM and 44.6 ± 6.2 SEM) and RAS (132.9 ± 26.4 SEM and 169.3 ± 55.0 SEM).
Figure 2. Antigen-specific IFN-γ producing CD8+ T cell responses in mice following a prime-boost vaccination with P. yoelii 17 XNL or P. berghei ANKA CAS or RAS.
The CD8+ T-cell response to a H-2Kd-specific CD8+ peptide (SYVPSAEQI) from the P. yoelii CSP (A) or P. berghei CSP (SYIPSAEKI ) (B) was measured by an IFN-γ ELISPOT assay of BALB/c mouse splenocytes. Grey bars represent the number of spot-forming cells per 106 splenocytes incubated with antigen-presenting cells loaded with CSP peptide. White bars represent the background control splenocytes incubated with unprimed antigen-presenting cells. Splenocytes were harvested from five (A) or three (B) mice and assayed individually. The experiment was completed in triplicate for each animal and the mean of all animals is shown for both groups. *Indicates significant difference: p=0.0031, ANOVA, n=5 (A) or p=0.0088, ANOVA, n=3 (B). There is no significant difference between the number of spot-forming cells in RAS and CAS in (A) or (B).
3.3 Immunization of mice with CAS induces sporozoite-specific antibodies
Antibodies recognizing Plasmodium sporozoites are produced in animals immunized with both RAS and GAS, which contribute to the generation of protective immunity [11-13]. We tested whether immunization with P. yoelii or P. berghei CAS induces parasite-specific antibodies using serum harvested from the same animals used in the ELISPOT assay. Animals immunized with P.yoelii or P. berghei RAS and CAS showed similar high antibody titers to whole sporozoites of the same species, in contrast to naïve mice (Fig. 3 A and B). Antibody titers were also examined in heterologous sporozoites. We found that sera from mice immunized with P. yoelii did not react with P. berghei sporozoites. When the reverse experiment was performed, only sera from some P. berghei immunized mice reacted at very high concentrations (1:100) with P. yoelii sporozoites (data not shown).
Figure 3. Specific antibody titers in mice to P. yoelii and P. berghei sporozoites following a prime-boost vaccination regimen with CAS or RAS.
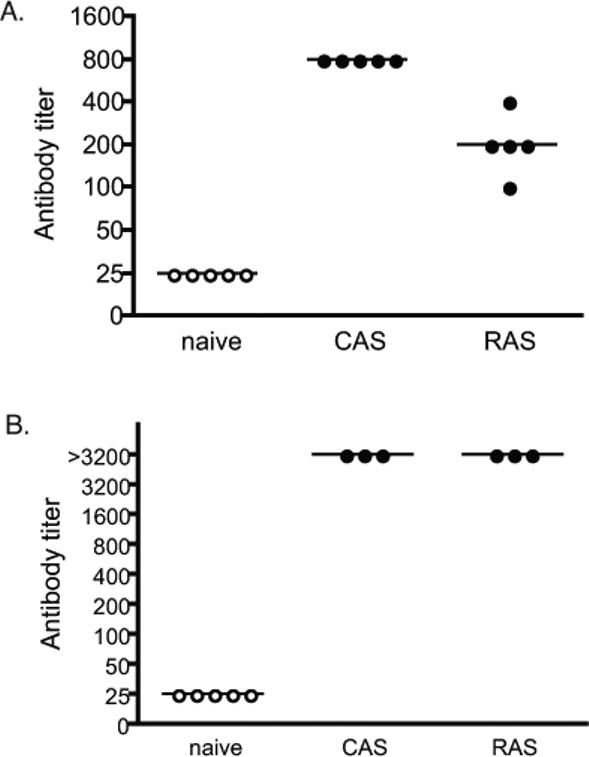
Sera from animals immunized with P. yoelii CAS or RAS was obtained and titers were determined by immunofluorescence using whole sporozoites from P. yoelii (A) or sera from animals immunized with P. berghei was used to determine titers and P. berghei sporozoites (B). Each circle represents data from one mouse. Black circles represent data where sporozoites were florescent, while open circles represent the lowest dilution of serum that did not produce florescent sporozoites. The experiment was completed in triplicate at each concentration for sera from each animal.
4. Discussion
This study shows that vaccination with P. yoelii CAS produce sterile protection in BALB/c mice, and that three doses of P. berghei CAS were sufficient to cross protect against heterologous challenge with P. yoelii sporozoites if challenged within 10 days. However, the cross-protecting immunity was short lived and was not observed after 21 days. Vaccination with CAS was as effective as RAS at producing sterile immunity against homologous and heterologous challenge with P. yoelii: heterologous protection has been described between P. berghei and P. yoelii RAS and GAS [23]. The generation of heterologous protection is important when considering the potential development of an attenuated sporozoite vaccine since due to the high multiplicity of infection in the field, protection needs to be generated against multiple strains of P. falciparum [24].
This study is the first characterization of the basic immune effector mechanisms induced by vaccination with centanamycin-attenuated Plasmodium parasites. Previous studies in BALB/c mice have identified H-2Kd-restricted major histocompatibility complex class I epitopes derived from CSP as important targets of protective immunity in mice vaccinated with RAS from both P. yoelii and P. berghei [12, 21, 25-27]. RAS and GAS generate immunity to sporozoite challenge, which is to a large extent mediated by IFN-γ producing CD8+ T cells [8, 11, 12]. Our results show that a similar CD8+ T cell response is induced by vaccination with CAS which, in addition induced an antibody response to whole sporozoites similar to that induced by RAS [28] and GAS [11, 12].
Interestingly, the compound centanamycin used in the chemical attenuation process may provide some advantages over other attenuation approaches. Attenuation of RAS presumably occurs due to double-strand breaks in the DNA that leads to a block in liver stage development [3]. Sporozoites treated with centanamycin, however, would contain a set of adducts covalently bound to adenine nucleotides [19] and the number of adducts can be defined bioinformatically [29, 30]. Given that over- and under-irradiation of sporozoites could impair the immunogenicity of RAS, CAS may have the advantage that the attenuation process can be strictly controlled to achieve saturation of the potential DNA binding sites. Free compound can be washed from the treated sporozoites before vaccination to minimize the risk of toxicity. In addition, a parasite that is attenuated by irradiation or non-saturating doses of centanamycin may produce sporozoites that arrest at various stages of development, producing a potentially more diverse immune response before the parasites are cleared from the liver. In fact, it was recently shown that the transcript and protein expression profiles of the Plasmodium liver stage change significantly during late development [31]. Genetic attenuation is likely to produce parasites that arrest at the same stage of development, when the function of an essential gene product is required.
In summary, the results reported here and those observed with CAS of P. berghei [14] demonstrate that chemical attenuation could complement other strategies for the generation of highly effective attenuated sporozoite vaccines. This study provides the basis on which to further explore the immune mechanisms induced by a CAS vaccine that protect the host from malaria.
Acknowledgements
We thank S. Gonzalez and J. Noonon for assistance with mosquito experiments. We thank Dr. A. Waters who kindly provided the P. berghei ANKA strain and Dr. Alberto Moreno for providing the CD8+ epitope P. berghei peptide. We thank Spirogen Ltd. for the use of centanamycin.
This work was supported by a Canada Graduate Scholarship, Natural Sciences and Engineering Research Council of Canada (L.P.); Centre for Host-Parasite Interactions Bridging Funds grant (L.P.); NIH grant RO1 AI (grant 053698, A.R.); Canadian Foundation for Innovation (grant 201221, T.S.); Le Fonds Québécois de la Recherche sure la Nature et les Technologies (FQRNT) Centre for Host-Parasite Interactions (grant 87902, T.S.); and a Canada Research Chair in Immunoparasitology (grant 201221, T.S.).
Abbreviations
- p.f.
post-feeding
- i.v.
intravenous
- CAS
chemically attenuated sporozoites
- RAS
radiation-attenuated sporozoites
- p.i.
post-infection
- SFC
spot-forming cells
- CSP
circumsporozoite protein
References
- 1.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002 Apr 15;185(8):1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 2.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967 Oct 14;216(5111):160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 3.Ward JF. DNA damage and repair. Basic life sciences. 1991;58:403–15. doi: 10.1007/978-1-4684-7627-9_15. discussion 15−21. [DOI] [PubMed] [Google Scholar]
- 4.Mellouk S, Lunel F, Sedegah M, Beaudoin RL, Druilhe P. Protection against malaria induced by irradiated sporozoites. Lancet. 1990 Mar 24;335(8691):721. doi: 10.1016/0140-6736(90)90832-p. [DOI] [PubMed] [Google Scholar]
- 5.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005 Jan 13;433(7022):164–7. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 6.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):3022–7. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12194–9. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jobe O, Lumsden J, Mueller AK, Williams J, Silva-Rivera H, Kappe SH, et al. Genetically Attenuated Plasmodium berghei Liver Stages Induce Sterile Protracted Protection that Is Mediated by Major Histocompatibility Complex Class I-Dependent Interferon- gamma -Producing CD8+ T Cells. J Infect Dis. 2007 Aug 15;196(4):599–607. doi: 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, Takagi A, et al. Protracted Sterile Protection with Plasmodium yoelii Pre-erythrocytic Genetically Attenuated Parasite Malaria Vaccines Is Independent of Significant Liver-Stage Persistence and Is Mediated by CD8+ T Cells. J Infect Dis. 2007 Aug 15;196(4):608–16. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- 10.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii Sporozoites with Simultaneous Deletion of P52 and P36 Are Completely Attenuated and Confer Sterile Immunity against Infection. Infect Immun. 2007 Aug;75(8):3758–68. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller AK, Deckert M, Heiss K, Goetz K, Matuschewski K, Schluter D. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am J Pathol. 2007 Jul;171(1):107–15. doi: 10.2353/ajpath.2007.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000 Aug 1;165(3):1453–62. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues M, Nussenzweig RS, Zavala F. The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology. 1993 Sep;80(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell LA, Yanow SK, Lee M, Spithill TW, Rodriguez A. Chemical attenuation of Plasmodium berghei sporozoites induces sterile immunity in mice. Infect Immun. 2008 Jan 3; doi: 10.1128/IAI.01399-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douradinha B, van Dijk MR, Ataide R, van Gemert GJ, Thompson J, Franetich JF, et al. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int J Parasitol. 2007 May 21; doi: 10.1016/j.ijpara.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Orjih AU, Cochrane AH, Nussenzweig RS. Comparative studies on the immunogenicity of infective and attenuated sporozoites of Plasmodium berghei. Trans R Soc Trop Med Hyg. 1982;76(1):57–61. doi: 10.1016/0035-9203(82)90019-0. [DOI] [PubMed] [Google Scholar]
- 17.Vanderberg J. The transmission by mosquitoes of Plasmodia in the laboratory. In: Krier J, editor. Malaria: pathology, vector studies and culture. Academic Press; New York: 1980. pp. 154–218. [Google Scholar]
- 18.Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004 Sep;137(1):23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Sato A, McNulty L, Cox K, Kim S, Scott A, Daniell K, et al. A novel class of in vivo active anticancer agents: achiral seco-amino- and secohydroxycyclopropylbenz[e]indolone (seco-CBI) analogues of the duocarmycins and CC-1065. J Med Chem. 2005 Jun 2;48(11):3903–18. doi: 10.1021/jm050179u. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho LH, Hafalla JC, Zavala F. ELISPOT assay to measure antigen-specific murine CD8(+) T cell responses. J Immunol Methods. 2001 Jun 1;252(1−2):207–18. doi: 10.1016/s0022-1759(01)00331-3. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Maryanski JL, et al. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. International immunology. 1991 Jun;3(6):579–85. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 22.Caro-Aguilar I, Lapp S, Pohl J, Galinski MR, Moreno A. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes and infection / Institut Pasteur. 2005 Oct;7(13):1324–37. doi: 10.1016/j.micinf.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Sedegah M, Weiss WW, Hoffman SL. Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites. Parasite Immunol. 2007 Nov;29(11):559–65. doi: 10.1111/j.1365-3024.2007.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richie T. High road, low road? Choices and challenges on the pathway to a malaria vaccine. Parasitology. 2006;133(Suppl):S113–44. doi: 10.1017/S0031182006001843. [DOI] [PubMed] [Google Scholar]
- 25.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989 Sep 28;341(6240):323–6. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 26.Sano G, Hafalla JC, Morrot A, Abe R, Lafaille JJ, Zavala F. Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J Exp Med. 2001 Jul 16;194(2):173–80. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrot A, Zavala F. Regulation of the CD8+ T cell responses against Plasmodium liver stages in mice. Int J Parasitol. 2004 Dec;34(13−14):1529–34. doi: 10.1016/j.ijpara.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Egan JE, Weber JL, Ballou WR, Hollingdale MR, Majarian WR, Gordon DM, et al. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–6. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- 29.Woynarowski JM, Krugliak M, Ginsburg H. Pharmacogenomic analyses of targeting the AT-rich malaria parasite genome with AT-specific alkylating drugs. Mol Biochem Parasitol. 2007 Jul;154(1):70–81. doi: 10.1016/j.molbiopara.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Yanow SK, Purcell LA, Lee M, Spithill TW. Genomics-based drug design targets the AT-rich malaria parasite: implications for antiparasite chemotherapy. Pharmacogenomics. 2007 Sep;8(9):1267–72. doi: 10.2217/14622416.8.9.1267. [DOI] [PubMed] [Google Scholar]
- 31.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A. 2008 Jan 8;105(1):305–10. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]