Abstract
This commentary integrates the most recent information on coronary heart disease from the WHI trial and our new large observational studies, which complement and expand the WHI results to the overall population. Breast and colon cancer findings are also considered.
Keywords: hormone replacement therapy, menopause, hysterectomy, cohort studies, randomized controlled trial, coronary heart disease
INTRODUCTION
Finding and applying the best evidence in practice is the goal of all clinicians and physician scientists. However, all clinical research has pitfalls and all study designs have strengths and limitations (1). Adding to the confusion is that at times evidence can be contradictory, or interpretation of evidence can change. A current example is the impact of estrogen and hormone therapy on menopausal women. Despite an overwhelming amount of literature in a very short time, our understanding of the effects of hormone therapy has rapidly and dramatically changed from a potential positive effect on cardiovascular disease to a harmful effect, and then to a possible protective effect in some women (2–9).
The purpose of this commentary is to put into perspective the current evidence concerning hormone therapy (HT), focusing on the recent re-analysis and expansion of the Women’s Health Initiative randomized controlled clinical trial (WHI RCT) data and new observational studies performed by our group (10–12), that may aid in convergence of the apparent conflicting results regarding the effect of estrogen only and combined hormone therapy for menopausal women.
Historical Background
The Nurses Health Study and other observational studies conducted in the 1990’s found that hormone therapy in post-menopausal women was associated with a 30 – 50% reduction in the development of coronary artery disease (2,3). These results along with the beneficial effects of estrogens such as relief of hot flashes, reduction in urogenital atrophy and an improvement in bone density, led to the widespread use of this therapy despite the increased risks of breast cancer and venous thromboembolic events. However, observational studies are considered to provide less convincing evidence than randomized trials since they are limited by potential bias and the inability to control for unidentified confounding factors. One theory specific to the observational studies regarding hormone replacement was “healthy women bias” where women who choose to take hormone therapy were inherently healthier than those that did not take the same therapy. Therefore, randomized clinical trials (RCTs) were conducted to test the hypothesized benefit of hormone therapy on cardiovascular disease.
In 1998, the first large RCT evaluating hormonal therapy, the Heart and Estrogen/progestin Replacement Study (HERS), reported that cardiovascular events were not altered in women with established coronary artery disease who took combined estrogen and progestin replacement therapy, despite widespread initial publicity of an increase in cardiovascular events in the first year after initiation of therapy (4). Subsequently the landmark WHI RCT, designed to assess the effect of hormone therapy as a primary prevention for cardiovascular disease, found that combined HT was not protective and actually increased the risk of coronary heart disease (CHD – the combined outcomes of myocardial infarction and coronary death) and produced similar results for myocardial infarction alone in women (5). Both these publications contrasted with the preponderance of observational trials reporting a beneficial effect of hormone therapy and precipitated a major change in the philosophy regarding the use of HT in post-menopausal women (2–6).
The US Preventive Services Task force considers evidence from a properly designed randomized trial level I evidence, as its design minimizes bias and confounding. However, RCTs suffer from the limitation that they are only applicable to the specific population included in the trial. The cohorts in the WHI RCT included a large percentage of women older than 70 years of age and very few women initiating therapy near the onset of menopause. Therefore the WHI RCT was subjected to criticism, especially as it may lack generalizability to younger women or women starting hormone therapy near the onset of menopause. Thus a vigorous debate about the use of HT ensued within the medical profession (6). Furthermore the changes in viewpoints over time have led to confusion in the public about the risks and benefits of HT.
Following the initial WHI RCT report which included women with an intact uterus treated with combined HT, the results of the second arm of the study, assessing a women with a prior hysterectomy treated solely with conjugated estrogens also showed no protective effect on CHD (HR 0.91 [0.75–1.12]): but did not exhibit the increase in cardiac disease reported initially in the WHI RCT with combined HT (HR 1.32 [1.02–1.74]) (5,7).
Evolution of the Interpretation of WHI data
Several attempts have been made to address the age and relationship to menopause shortcomings of the WHI trial. Re-analysis of the WHI data suggests that women who start treatment with combined HT under age 70 years, or less than 20 years from the onset of menopause, do not exhibit an increase in CHD (Table 1) (8). There also was a suggestion that treatment with estrogen alone might decrease CHD in women aged 50 – 59 years or when used less than 10 years after the onset of menopause; however, this analysis was not significant statistically (Table 2).
TABLE 1.
WHI RCT and GPRD STUDIES IN WOMEN WITH AN INTACT UTERUS TREATED WITH COMBINED HORMONE RECPLACEMENT THERAPY
| WHI RCTa (50 – 79 yrs) |
WHI RCTb (50 – 70 yrs) |
WHI RCTb (50 – 59 yrs) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatede | Placeboe | HR (95% CI)f | Treatede | Placeboe | HR (95% CI)f | Treatede | Placeboe | HR (95% CI)f | |
| Estrogen & Progestin | Estrogen & Progestin | Estrogen & Progestin | |||||||
| (n) | 8506 | 8102 | 6692 | 6340 | 2839 | 2683 | |||
| CHDj | 164 | 122 | 1.32 [1.02–1.74] | 116 | 99 | 1.11 [0.84–1.47] | 38 | 27 | 1.29 [0.79–2.12] |
| CVAk | 127 | 85 | 1.41 [1.07–1.85] | 98 | 64 | 1.45 [1.05–2.02] | 26 | 16 | 1.41 [0.75–2.65] |
| DEATH | 231 | 218 | 0.98 [0.82–1.18] | 146 | 141 | 0.98 [0.77–1.25] | 35 | 47 | 0.69 [0.44–1.07] |
| GPRD STUDYc (55 – 79 yrs) |
GPRD STUDYd (50 – 55 yrs) |
||||||||
| Expg | UnExph | Adj HR (95% CI)i | Expg | UnExph | Adj HR (95% CI)i | ||||
| Estrogen & Progestin | Estrogen & Progestin | ||||||||
| (n) | 13,658 | 37,730 | 20,654 | 30,102 | |||||
| MI | 141 | 491 | 0.95 [0.78–1.16]L | 96 | 150 | 0.91 [0.69–1.20]n | |||
| CVA | 138 | 405 | 1.23 [0.99–1.52]m | 101 | 109 | 1.52 [1.11–2.08]o | |||
| DEATH | 317 | 1378 | 0.75 [0.65–0.86] | 231 | 429 | 0.76 [0.65–0.91] | |||
Reference (5), 21.5% of subjects > age 70 vs. 2.5% in GPRD study.
Reference (8)
Reference (10)
Reference (11)
Number of events
Hazard ratio (95% confidence interval)
Exposed, number of events
Unexposed, number of events
Cox adjusted hazard ratio (95% confidence interval)
Coronary Heart Disease (CHD) a combination of myocardial infarction and coronary death was the primary outcome in the WHI RCT; however the results for myocardial infarction alone were similar. Results in the GPRD studies are all acute myocardial infarctions (MI).
Cerebrovascular accident
Adjusted hazard ratio using Prior event rate ratio (PERR) = 1.40 (0.87 – 2.44)
Adjusted hazard ratio using PERR = 2.63 (1.38 – 7.43)
Adjusted hazard ratio using PERR = 0.77 (0.41–1.42)
Adjusted hazard ratio using PERR = 2.20 (1.12–5.45)
TABLE 2.
WHI RCT AND GPRD STUDIES in WOMEN WITH A PRIOR HYSTERECTOMY TREATED WITH ESTROGEN ONLY
| WHI RCTa (50 – 79 yrs) |
WHI RCTb (50 – 70 yrs) |
WHI RCTb (50 – 59 yrs) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treated | Placebo | HR (95% CI) | Treated | Placebo | HR (95% CI) | Treated | Placebo | HR (95% CI) | |
| Estrogen only | Estrogen only | Estrogen only | |||||||
| (n) | 5310 | 5429 | 4024 | 4128 | 1637 | 1673 | |||
| CHD | 177 | 199 | 0.91 [0.75–1.12] | 117 | 140 | 0.86 [0.67–1.11] | 21 | 34 | 0.63 [0.36–1.09] |
| CVA | 158 | 118 | 1.39 [1.10–1.77] | 102 | 75 | 1.40 [1.03–1.91] | 18 | 21 | 0.89 [0.47–1.69] |
| DEATH | 291 | 289 | 1.04 [0.88–1.22] | 163 | 179 | 0.94 [0.75–1.16] | 34 | 48 | 0.71 [0.46–1.11] |
| GPRD STUDYc (55 – 79 yrs) |
GPRD STUDYc (50 – 55 yrs) |
||||||||
| Exp | UnExp | Adj HR (95% CI) | Exp | UnExp | Adj HR (95% CI) | ||||
| Estrogen only | Estrogen only | ||||||||
| (n) | 6890 | 11,572 | 6842 | 9624 | |||||
| MI | 59 | 238 | 0.50 [0.38–0.67]d | 39 | 66 | 0.85 [0.57–1.37]f | |||
| CVA | 93 | 191 | 0.95 [0.74–1.23]e | 52 | 72 | 1.10 [0.76–1.58]g | |||
| DEATH | 182 | 497 | 0.68 [0.57–0.81] | 65 | 169 | 0.53 [0.40–0.71] | |||
The protective effect in younger women is supported further by the recent finding that women 50 – 59 years old in the original WHI study of women with hysterectomy treated solely with estrogen exhibited less coronary calcification than the women receiving placebo (9). However, interpretation of this data should be cautious because it evaluated women taking estrogen only, was not randomized and coronary artery calcification is a surrogate endpoint.
Attempts to draw conclusions concerning CHD from this WHI re-analysis beyond those already noted are not convincing (8). This especially holds for the analyses that combined the data using subjects from both arms of the WHI who were taking either combined HT or estrogen-only, which was done in order to increase the statistical power of the analysis. As discussed subsequently, given the major differences between the results of the combined HT and estrogen only components of the WHI, combining this data is not justifiable.
Recent population-based observational studies
Women with a uterus taking combined hormone therapy
Additional relevant data emerged from our group’s investigations of the WHI and other RCT’s. In a series of studies, data from a primary care electronic medical record database were used to determine how well observational studies would replicate the findings of previously performed RCT’s (10–13). These investigations, designed to mimic the RCT’s to the extent feasible except for randomization of treatment, used the United Kingdom General Practice Research Database (GPRD), which contains a representative sample of approximately 5.5% of the United Kingdom population with approximately 8.0 million patient records.
The GPRD observational studies simulating the WHI RCT are particularly informative since they provide a representative sample of the overall population, encompass a large number of young women, and have incorporated a new analytic technique that appears to address the problem of unrecognized confounding (vide infra), a major impediment to the validity of observational studies (10–14). They are summarized herein and compared with the results of the WHI RCT (10–12).
Although the study employing the GPRD used similar inclusion and exclusion criteria, precise replication of the age profile in the WHI RCT was not possible, since prescription of hormonal therapy occurred infrequently for women older than 70 years in the overall population. For example, only 2.5 % of women in the GPRD treated with HT were older than 70 years as contrasted with 21.5% in the WHI RCT. To overcome this limitation we compared the GPRD study both to the overall WHI RCT and also to the WHI subgroup aged 50–70 years. It should be noted that the WHI RCT used conjugated equine estrogen and daily medroxyprogesterone; whereas the women in GPRD study used conjugated equine estrogen and norgestrel taken on last 11 days of the cycle (10).
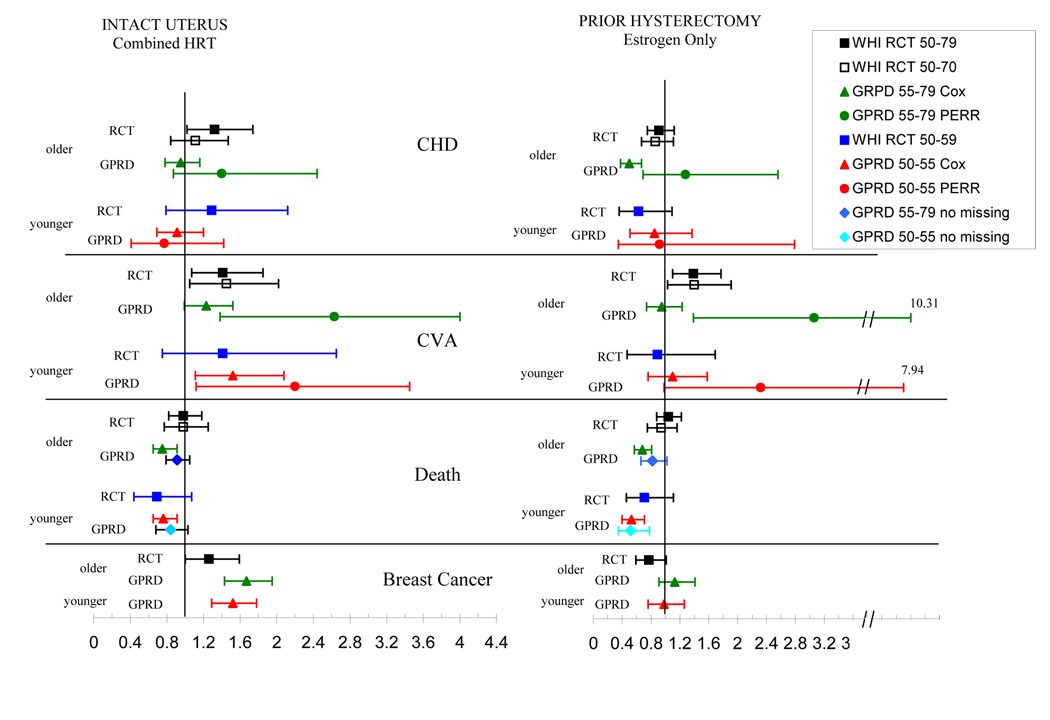
A comparison of our results in women with an intact uterus treated with combined HT and those of the WHI are presented in Table 1, and Figure 1. The WHI RCT reported CHD as the primary outcome, but the results for myocardial infarction were similar. Myocardial infarction only was assessed in the GPRD studies. Myocardial infarction was not decreased by treatment with combined HT in the GPRD study, in contrast to the decrease reported in most prior observational studies (2,3,6). The GPRD study also did not show an increase in myocardial infarction as was reported in the original WHI publication (5). However, the GPRD study results are similar to the results of the recent age re-analysis of the WHI RCT, which did not find an increase in CHD in women younger than age 70 (8).
Figure 1.
Comparison of the response to HT between the WHI RCT and our Population Studies. The response of women with an intact uterus treated with combined HT is shown in the left panel, and those with a prior hysterectomy treated with estrogen only is shown on the right. The data for each outcome is grouped with the response of older women in the upper and younger women in the lower portion.
Abbreviations: CHD – coronary heart disease in WHI RCT or myocardial infarction only in GPRD studies, CVA – cerebrovascular accident.
The legend defines the age of the study group. Cox adjusted HR’s are shown for all data except those labeled PERR, which were analyzed with this adjustment. “Missing data” in the death analysis refers to a subset of the cohort that was not missing any data on the potential confounders – blood pressure, BMI, and smoking.
The WHI re-analysis also reported the results for women ages 50 – 59, or less than 10 years after the onset of menopause, and found no significant change in CHD in this subgroup (8). However, the number of subjects studied was limited. Our second GPRD study was purposefully limited to women ages 50 –55 years and had a greater number of subjects than the WHI. These results demonstrated no protective or adverse effect of combined HT on the occurrence of myocardial infarction (11) consistent with the WHI analysis of younger women. Of note, and interest was that the positive association of combined HRT and stroke was consistent in both the WHI and GPRD studies in both older and younger women.
Perhaps the most meaningful outcome of a clinical trial evaluating a therapy is death. The WHI RCT found that death was unchanged in older women treated with combined HT, but data suggest that younger women treated with combined HT might have decreased mortality. In the GPRD study death appeared to decrease in both the older and younger women treated with combined HT; however, the conclusion based on the GPRD data in this study should be tempered by the fact that a large percentage of subjects were missing baseline data on several important confounders (such as smoking, BMI and blood pressure). Analysis of the GPRD subgroup “No Missing” that excluded subjects missing this data did not exhibit a decrease in death whereas all other outcomes were similar to those in the overall cohort (Fig 1).
Women with a hysterectomy taking estrogen therapy
In both the WHI RCT and our GPRD studies the response to conjugated estrogen alone in women with a prior hysterectomy (Table 2 and Figure 1) differs from the findings with combined HT in women with an intact uterus (7,12). It is important to note that our GPRD study confirms the finding of the WHI that women with a prior hysterectomy have a baseline health profile exhibiting both an increased prior occurrence of CHD and an increased risk for the development of CHD when compared to women who have not had a hysterectomy (7,12). It is not possible to yet determine if the difference in the response to HT in the women with and without a hysterectomy is influenced by the addition of progestin treatment and/or the difference in the cardiac risk profile.
In contrast to women treated with combined HT, in the WHI RCT of women treated with estrogen-only HT CHD was not increased in the overall population or the subset of women older than age 70 or more than 20 years post menopause. Furthermore there was a trend towards decreased CHD in women ages 50–59, but these results were not significant statistically possibly due to limited power from the relatively small number of women in this subgroup (8).
In the GPRD study of estrogen-only treated older women the Cox adjusted HR for myocardial infarction was decreased. However, as discussed below, the results appear to have been influenced by “unmeasured confounding”. In the GPRD study of younger estrogen-treated women with a hysterectomy, the Cox adjusted HR was not decreased significantly, did not differ significantly from the WHI results in women aged less than 60 years, and did not exhibit evidence for unmeasured confounding.
The risk of cerebrovascular accident (CVA) was increased in the WHI RCT of older estrogen-only treated women, which was similar to the results found in women treated with combined HT. In the GPRD study of older women although the Cox adjusted HR for CVA was not increased, there is reason to believe that unmeasured confounding might account for this finding in a fashion similar to the results for myocardial infarction In younger women, the data on the risk of stroke are not conclusive in either the WHI or GPRD studies.
Death was not altered significantly in the WHI RCT for the entire population of women treated with estrogen only. However, mortality may be decreased in younger women treated with estrogen only, similar to the finding of younger women treated with combined hormone therapy described above. A combined analysis of both WHI studies in younger women yielded a significant decrease in death; however, given the differences between the effects of combined HT and estrogen-only therapy the justification for combining this data is not convincing (8,12). In the GPRD studies, death was decreased for women taking estrogen only and combined therapy. The decrease in death persisted in the younger women, even in those with “no missing data”. The concordance of our data and the findings of the WHI RCT more confidently suggested that death might be decreased in younger women with a hysterectomy treated with estrogen alone.
Adjustment for unmeasured confounding
Prior observation studies have been limited by possible bias due to unmeasured confounding. We have recently devised an analytic approach that adjusts for all confounding, called the Prior Event Rate Ratio (PERR) adjustment (13,14). It assumes that the ratio of the Exposed to Unexposed event rates for a specific outcome prior to the study start date reflects the combined effect of all confounders (both measured and unmeasured) independent of any influence of treatment, provided that neither the subsequently Exposed or Unexposed groups ingested the drug undergoing study prior to the study start date. Thus when the unadjusted hazard ratio (HR) during the study is divided by the PERR, this adjusted HR corrects for all confounding.
The PERR adjustment has been used in the evaluation of other GPRD-RCT comparative studies evaluating cardiovascular outcomes and exhibited remarkable success to adjust findings that appeared to be affected by unmeasured confounding (13,14). When unmeasured confounding exists the PERR adjusted HR differs significantly from the Cox adjusted HR. Use of this analysis in our studies of hormone therapy did not suggest the presence of unmeasured confounding for either the myocardial infarction or CVA outcomes in the combined HT studies, nor in the younger estrogen- treated women with a hysterectomy (Fig 1). However PEER adjustment demonstrated a significantly increased HR (compared to the Cox adjusted HR) in older women who took estrogen only for the outcomes of myocardial infarction and CVA. The upward adjustment of the HR suggests the presence of unmeasured confounding (Fig 1). The precise PERR adjusted HR must be interpreted cautiously in these GPRD studies designed to replicate the WHI RCT, since the low number or prior events resulted in a wide confidence interval.
Evaluation of other outcomes
It is also pertinent to comment upon the findings related to breast cancer risk in both the WHI and GPRD studies. As shown in figure 1, breast cancer was increased in both the older and younger women treated with combined HT in the GPRD studies, similar to the findings in the WHI RCT. Furthermore breast cancer risk was unchanged in both the older and younger women with a prior hysterectomy treated with estrogen only in the GPRD studies, similar to the results in the WHI RCT. Additional analyses of women in both the combined HT and estrogen only GPRD studies that were not exposed to HT at any time prior to the start of the study, yielded results for breast cancer similar to the overall study cohorts.
As reported in our primary publications other outcomes, including colorectal cancer and hip fracture, largely were similar in the GPRD and WHI studies for both the comparisons of combined HT and estrogen only therapy (10–12). Venous thromboembolic events also were similar in the studies of combined hormonal therapy, but probably suffered from unmeasured confounding in the GPRD estrogen-only study, as was found for myocardial infarction and CVA.
Summary
There is an almost overwhelming amount of data assessing the impact of hormonal therapy for menopausal women. When taken in aggregate the data may not be as disparate as it may seem. Data from the study of large segments of the population (i.e. the GPRD data) and randomized clinical trials (i.e. the WH) indicate that treatment with combined HT of women less than 70 years old or less than 20 years post-menopause neither protects against nor increases the risk for development of coronary heart disease. This conclusion is applicable to younger women ages 50–55 treated in the early stage of menopause. Conversely, data also strongly suggests that initiation of combined HT at more than 70 years of age or more than 20 years after the onset of menopause increases the risk of CHD (8). Also consistent is the finding that combined HT is associated with an increase risk for development of stroke and breast cancer regardless of age of therapy. Although the effect on overall mortality is not clear, the combination of the findings from the GPRD studies and the WHI RCT demonstrate that death is not increased by combined HT and that there may be a trend toward lower mortality in younger women.
Women with a prior hysterectomy treated with conjugated equine estrogen only, respond differently than those with an intact uterus treated with combined HT. Whether the differences are due to progestin treatment and/or the disparate cardiovascular risk profiles of women who have had a prior hysterectomy is not resolved. Coronary heart disease is not increased in women of any age with a prior hysterectomy treated with estrogen-alone and it may be decreased in younger women started on therapy within 10 years after menopause. Stroke is increased by treatment in older women but the effect on women younger than age 60 is unclear. In contrast to treatment with combined HRT, breast cancer is not increased in estrogen-only treated women during the 5 year follow-up period of these studies. Finally, overall mortality may be decreased in younger estrogen treated women.
Clinical implications
The indication for HT is management of the symptoms or consequences resulting form estrogen depletion after menopause. The therapeutic efficacy of HT to relieve vasomotor symptoms and treat genital atrophy, and reduce osteoporotic fractures is undisputed. Risks of hormonal therapy have been evident in observational studies, the WHI, and our large scale population analyses. The magnitude of this risk has not varied dramatically among these study type or over the years. The evolution of the association of HT and cardiovascular risk from protection to harm and now to possible protection again, has resulted in controversy and confusion. Like in many aspects of life, a pendulum often swings to extremes, but with time ends up resting in the middle.
HT is usually prescribed for a woman aged 45 – 60 years experiencing vasomotor symptoms. Hormone replacement is not indicated for cardioprotection for women in their 70’s, or for women who do not suffer from vasomotor symptoms or urogenital atrophy. Data clearly demonstrate, however, that clinicians can prescribe, and women can use, HT confidently during the time when therapy is most needed. The debate regarding potential benefits of HT on cardiovascular risk and/or mortality for women who start therapy in proximity to the menopausal transition is not resolved. The benefit and risk of any therapy, including HT, should be reassessed periodically in each individual patient and based on future scientific evidence.
Acknowledgments
RESEARCH SUPPORT: RO1-HL 073911 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: None of the authors have any conflicts of interest concerning the information reported in this manuscript.
CAPSULE
When new analyses of the WHI results are integrated with new observational studies, estrogen-progestin treatment has no adverse effects on coronary heart disease in women less than 70 years old.
REFERENCES
- 1.Barnhart KT, Sammel M. The truth is out there: A guide to the pitfalls of interpreting evidence based medicine in studies of human reproduction. Fertil Steril. 2002;77(2):223–228. doi: 10.1016/s0015-0282(01)03207-1. [DOI] [PubMed] [Google Scholar]
- 2.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. J Engl J Med. 1996;335:453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 3.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfel MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 4.Hulley S, Grady D, Bush T, Furbert C, Herrington D, Riggss B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 5.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in health postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 7.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Matthew AA, Rossouw JE, Carr J, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 10.Tannen RL, Weiner MG, Xie D, Barnhart K. A simulation using data from a primary care practice database closely replicated the women’s health initiative trial. Journal of Clinical Epidemiology. 2007;60:686–695. doi: 10.1016/j.jclinepi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Weiner MG, Barnhart K, Xie D, Tannen RL. Hormone replacement therapy and coronary heart disease in young women. Menopause. 2008;15:86–93. doi: 10.1097/gme.0b013e3180413e45. [DOI] [PubMed] [Google Scholar]
- 12.Tannen RL, Weiner MG, Xie D, Barnhart K. Estrogen affects postmenopausal women differently than estrogen plus progestin therapy. Human Reproduction. 2007;22:1769–1777. doi: 10.1093/humrep/dem031. [DOI] [PubMed] [Google Scholar]
- 13.Weiner MG, Xie D, Tannen RL. Replication of the Scandinavian Simvastatin Survival Study using a primary care medical record database prompted exploration of a new method to address unmeasured confounding. Pharmacoepidemiology and Drug Safety. 2008 doi: 10.1002/pds.1585. ( http://www.interscience.wiley.com) [DOI] [PubMed]
- 14.Tannen RL, Weiner MG, Xie D. Replicated studies of two randomized trials of angiotensin converting enzyme inhibitors: further empiric validation of the “prior event rate ratio” to adjust for unmeasured confounding by indication. Pharmacoepidemiology and Drug Safety. 2008 doi: 10.1002/pds.1584. ( http://www.interscience.wiley.com) [DOI] [PubMed]