Abstract
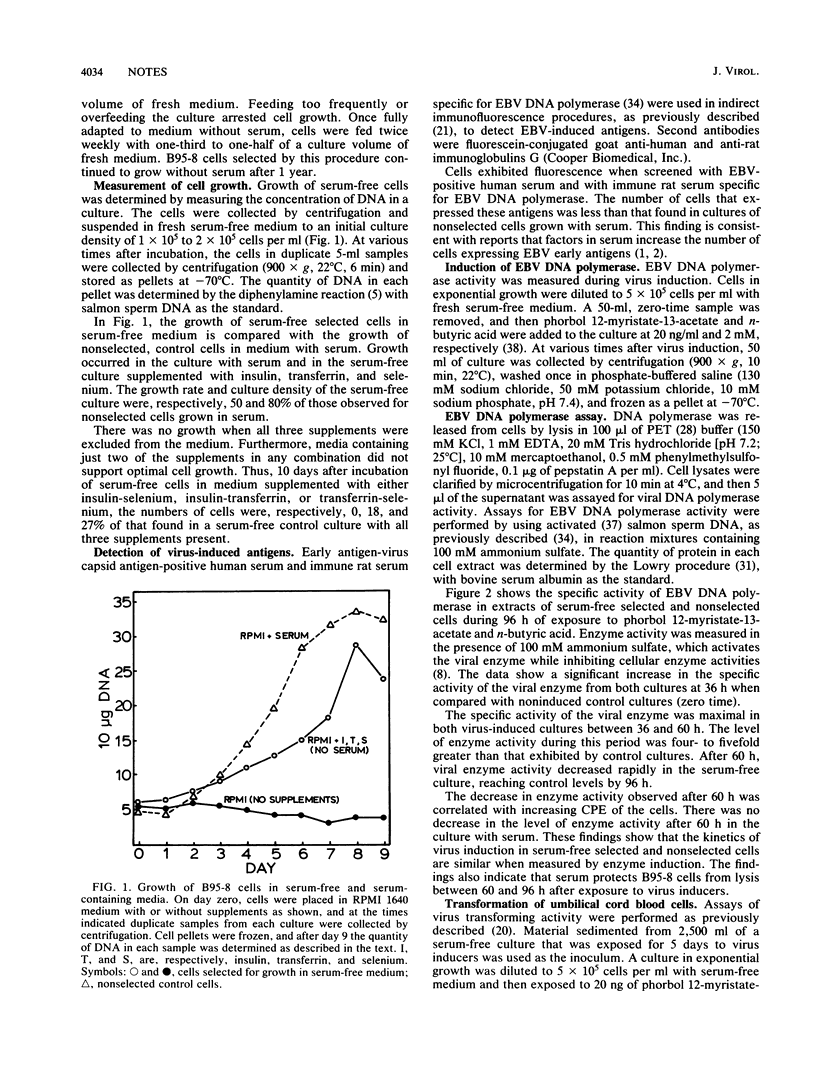
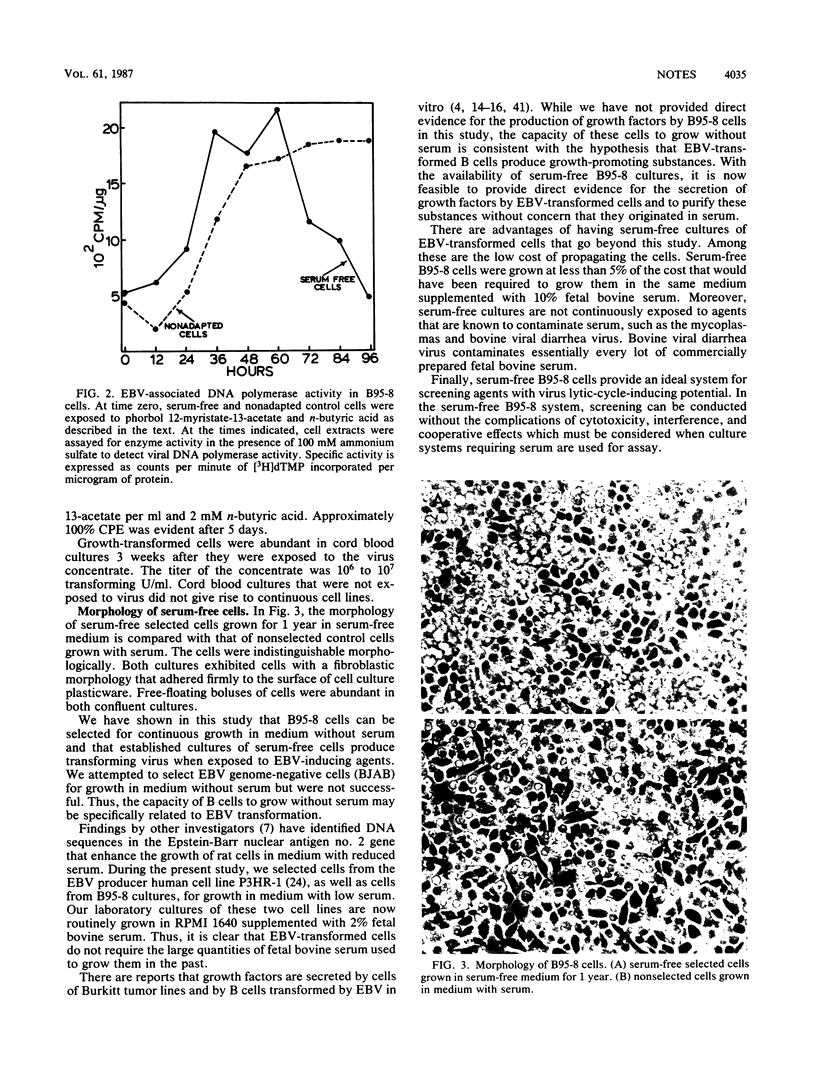
Epstein-Barr virus (EBV)-transformed tamarin (Saguinus oedipus) cells (B95-8) were selected for growth in medium with reduced serum and then transferred to serum-free medium which consisted of RPMI 1640 supplemented with insulin, transferrin, and selenium. Serum-free cells in continuous passage for 1 year had a morphology, growth rate, and culture density which approached those of B95-8 cells grown with serum. The cells expressed virus-induced antigens, including the EBV-associated DNA polymerase. Cells exposed to EBV-inducing agents, n-butyric acid and phorbol 12-myristate-13-acetate, produced transforming virus with titers comparable to those of cultures grown with serum. These findings demonstrate that serum is neither required for the growth of B95-8 cells nor necessary for induction or full expression of the EBV lytic phase in these cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Höfler P., Simon M. Epstein-Barr virus induction by a serum factor. Purification of a high molecular weight protein that is responsible for induction. J Biol Chem. 1982 Oct 10;257(19):11405–11410. [PubMed] [Google Scholar]
- Bauer G., Höfler P., Zur Hausen H. Epstein-Barr virus induction by a serum factor. I. Induction and cooperation with additional inducers. Virology. 1982 Aug;121(1):184–194. doi: 10.1016/0042-6822(82)90128-3. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson S. A., Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981 Aug 15;28(2):131–135. doi: 10.1002/ijc.2910280204. [DOI] [PubMed] [Google Scholar]
- Blazar B. A., Sutton L. M., Strome M. Self-stimulating growth factor production by B-cell lines derived from Burkitt's lymphomas and other lines transformed in vitro by Epstein-Barr virus. Cancer Res. 1983 Oct;43(10):4562–4568. [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Wang F., Hennessy K., Woodland E., Rickinson A., Kieff E. Expression of the Epstein-Barr virus nuclear protein 2 in rodent cells. J Virol. 1986 Aug;59(2):453–462. doi: 10.1128/jvi.59.2.453-462.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- Fu S. M., Hurley J. N., McCune J. M., Kunkel H. G., Good R. A. Pre-B cells and other possible precursor lymphoid cell lines derived from patients with X-linked agammaglobulinemia. J Exp Med. 1980 Dec 1;152(6):1519–1526. doi: 10.1084/jem.152.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Aman P., Rosén A., Ernberg I., Ehlin-Henriksson B., Klein G. Capacity of B-lymphocytic lines of diverse tumor origin to produce and respond to B-cell growth factors: a progression model for B-cell lymphomagenesis. Int J Cancer. 1985 Feb 15;35(2):251–256. doi: 10.1002/ijc.2910350218. [DOI] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., Aman P., Hughes-Jones N. C. Soluble factor requirements for the autostimulatory growth of B lymphoblasts immortalized by Epstein-Barr virus. J Exp Med. 1984 May 1;159(5):1554–1559. doi: 10.1084/jem.159.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., English L. S., Hughes-Jones N. C. Immortalized B lymphocytes produce B-cell growth factor. Nature. 1984 Jul 12;310(5973):145–147. doi: 10.1038/310145a0. [DOI] [PubMed] [Google Scholar]
- Grogan E., Jenson H., Countryman J., Heston L., Gradoville L., Miller G. Transfection of a rearranged viral DNA fragment, WZhet, stably converts latent Epstein-Barr viral infection to productive infection in lymphoid cells. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1332–1336. doi: 10.1073/pnas.84.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Walker J. L. Synthesis of Epstein-Barr virus after activation of the viral genome in a "virus-negative" human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine (thymidine kinase-virus antigen-immunofluorescence-herpesvirus fingerprints). Proc Natl Acad Sci U S A. 1972 Jan;69(1):78–82. doi: 10.1073/pnas.69.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Falk K., Ernberg I. Epstein-Barr virus transformation of human pre-B cells. J Exp Med. 1983 Aug 1;158(2):616–622. doi: 10.1084/jem.158.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Miller G., Robinson J., Heston L. Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology. 1977 Jan;76(1):152–163. doi: 10.1016/0042-6822(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. Effect of arginine-deficient media on the herpes-type virus associated with cultured Burkitt tumor cells. J Virol. 1968 Mar;2(3):182–191. doi: 10.1128/jvi.2.3.182-191.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kawanishi M., Harayama T., Takabayashi S. Combined effect of the extracts from Croton tiglium, Euphorbia lathyris or Euphorbia tirucalli and n-butyrate on Epstein-Barr virus expression in human lymphoblastoid P3HR-1 and Raji cells. Cancer Lett. 1981 Apr;12(3):175–180. doi: 10.1016/0304-3835(81)90066-5. [DOI] [PubMed] [Google Scholar]
- Ito Y., Yanase S., Fujita J., Harayama T., Takashima M., Imanaka H. A short-term in vitro assay for promoter substances using human lymphoblastoid cells latently infected with Epstein-Barr virus. Cancer Lett. 1981 Jun;13(1):29–37. doi: 10.1016/0304-3835(81)90083-5. [DOI] [PubMed] [Google Scholar]
- Joncas J., Boucher J., Boudreault A., Granger-Julien M. Effect of hydrocortisone on cell viability, Epstein-Barr virus genome expression, and interferon synthesis in human lymphoblastoid cell lines. Cancer Res. 1973 Sep;33(9):2142–2148. [PubMed] [Google Scholar]
- Kallin B., Sternås L., Saemundssen A. K., Luka J., Jörnvall H., Eriksson B., Tao P. Z., Nilsson M. T., Klein G. Purification of Epstein-Barr virus DNA polymerase from P3HR-1 cells. J Virol. 1985 May;54(2):561–568. doi: 10.1128/jvi.54.2.561-568.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katamine S., Otsu M., Tada K., Tsuchiya S., Sato T., Ishida N., Honjo T., Ono Y. Epstein-Barr virus transforms precursor B cells even before immunoglobulin gene rearrangements. Nature. 1984 May 24;309(5966):369–372. doi: 10.1038/309369a0. [DOI] [PubMed] [Google Scholar]
- Kawanishi M., Ito Y. Effect of short-chain fatty acids on Epstein-Barr virus early and viral capsid antigen induction in P3HR-1 cells. Cancer Lett. 1980 Dec;11(2):129–132. doi: 10.1016/0304-3835(80)90103-2. [DOI] [PubMed] [Google Scholar]
- Kawanishi M., Sugawara K., Ito Y. Epstein-Barr virus-induced early polypeptides in Raji and NC37 cells activated by diterpene ester TPA in combination with N-butyrate. Virology. 1981 Dec;115(2):406–409. doi: 10.1016/0042-6822(81)90123-9. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit R. G., Leung K., Shaw J. E. Specific immune serum to the Epstein-Barr virus DNA polymerase. J Virol. 1987 Oct;61(10):3331–3334. doi: 10.1128/jvi.61.10.3331-3334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Heston L., Miller G. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2762–2766. doi: 10.1073/pnas.80.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Mizuno F., Osato T. Epstein-Barr virus-associated antigens in non-producing clones of human lymphoblastoid cell lines. Nat New Biol. 1972 Oct 25;239(95):242–243. doi: 10.1038/newbio239242a0. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Lenoir G., Begon-Lours J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature. 1978 Nov 16;276(5685):270–272. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- Wakasugi H., Rimsky L., Mahe Y., Kamel A. M., Fradelizi D., Tursz T., Bertoglio J. Epstein-Barr virus-containing B-cell line produces an interleukin 1 that it uses as a growth factor. Proc Natl Acad Sci U S A. 1987 Feb;84(3):804–808. doi: 10.1073/pnas.84.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Katsuki T., Hinuma Y., Hoshino H., Miwa M., Fujiki H., Sugimura T. Induction of Epstein-Barr virus by an new tumor promoter, teleocidin, compared to induction by TPA. Int J Cancer. 1981 Aug 15;28(2):125–129. doi: 10.1002/ijc.2910280203. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., zur Hausen H. Induction of Epstein-Barr virus early antigens by intercalating chemicals in B95-8 cells. Virology. 1981 Dec;115(2):390–394. doi: 10.1016/0042-6822(81)90120-3. [DOI] [PubMed] [Google Scholar]
- Yoshie O., Tada K., Ono Y. Immunoglobulin production in Epstein-Barr virus-induced human monoclonal lymphoblastoid cell lines. Tohoku J Exp Med. 1980 Dec;132(4):397–403. doi: 10.1620/tjem.132.397. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Bornkamm G. W., Schmidt R., Hecker E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):782–785. doi: 10.1073/pnas.76.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]



