Abstract
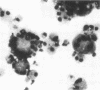
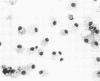
Ruminant lentiviruses share genomic sequences and biologic properties with human immunodeficiency viruses. Four ovine lentivirus strains were assessed for cytopathic effects and virus replication. Lentivirus isolate H/24 produced high virus titers and lysis of synovial cells but replicated slowly and caused no fusion of alveolar macrophages. Lentivirus isolates 84/28 and 85/14 produced low virus titers, less syncytia, and limited or no cell lysis in synovial cells and macrophages. In contrast, ovine lentivirus isolate 85/34 produced early peak virus titers and caused rapid fusion and lysis of both macrophages and synovial cells. Ovine lentivirus isolates which were cytopathic for macrophages induced lymphoproliferative disease when inoculated into lambs.
Full text
PDF
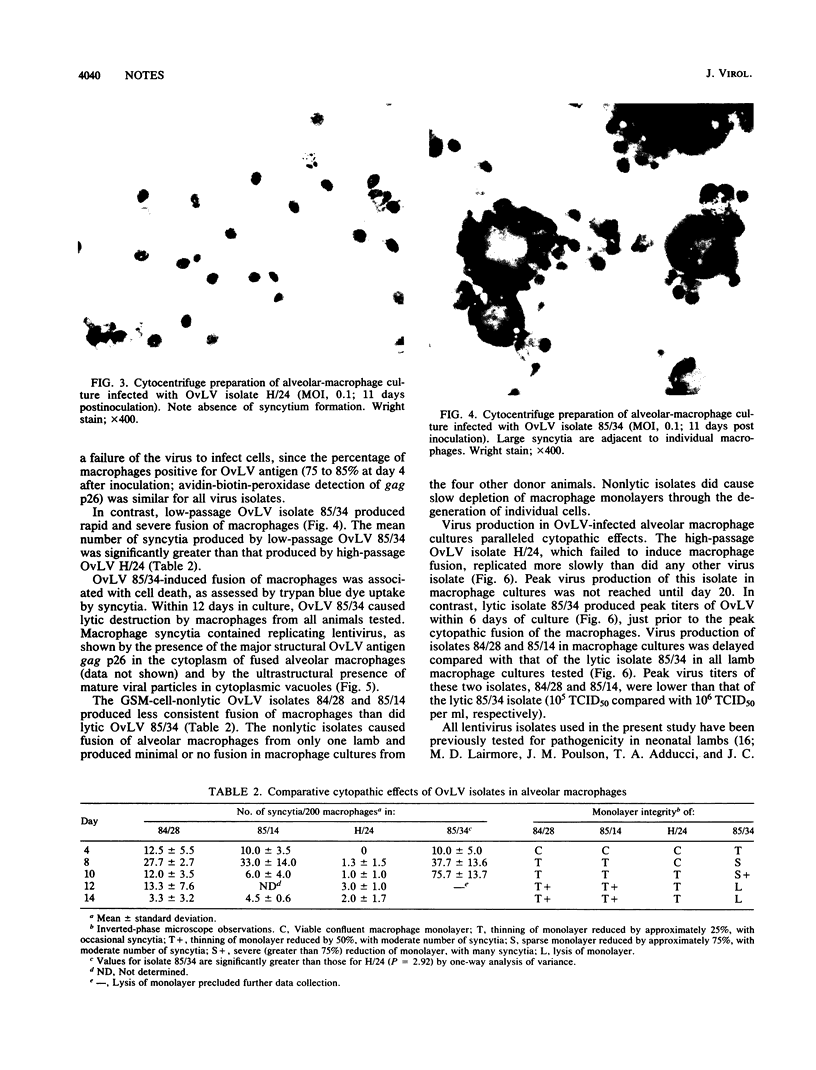
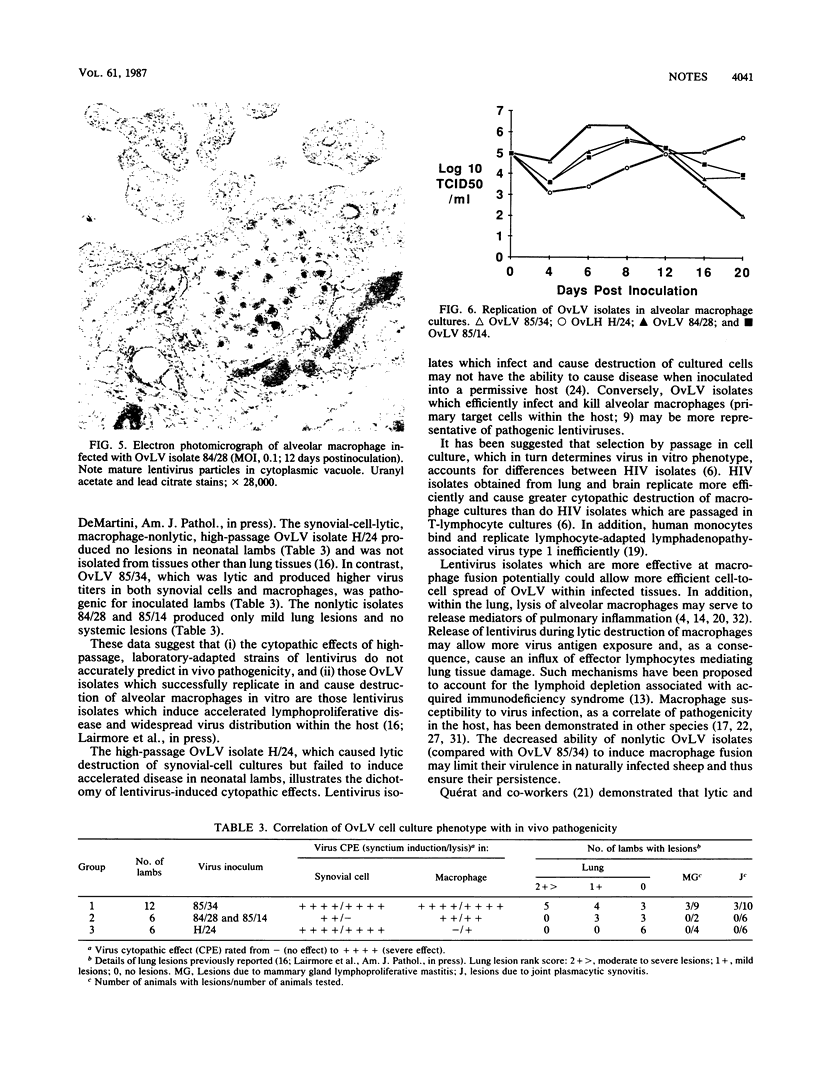

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis J. L., Molineaux S., Clements J. E. Visna virus exhibits a complex transcriptional pattern: one aspect of gene expression shared with the acquired immunodeficiency syndrome retrovirus. J Virol. 1987 May;61(5):1325–1331. doi: 10.1128/jvi.61.5.1325-1331.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini J. C., Rosadio R. H., Sharp J. M., Russell H. I., Lairmore M. D. Experimental coinduction of type D retrovirus-associated pulmonary carcinoma and lentivirus-associated lymphoid interstitial pneumonia in lambs. J Natl Cancer Inst. 1987 Jul;79(1):167–177. [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Immunomorphologic and morphometric changes in pulmonary lymph nodes of sheep with progressive pneumonia. Vet Pathol. 1985 Jan;22(1):32–41. doi: 10.1177/030098588502200105. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Ovine interleukin-2: partial purification and assay in normal sheep and sheep with ovine progressive pneumonia. Vet Immunol Immunopathol. 1985 Jan;8(1-2):15–25. doi: 10.1016/0165-2427(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Evans L. A., McHugh T. M., Stites D. P., Levy J. A. Differential ability of human immunodeficiency virus isolates to productively infect human cells. J Immunol. 1987 May 15;138(10):3415–3418. [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Levinson W. Inhibition of RNA slow viruses by thiosemicarbazones. Biochem Biophys Res Commun. 1973 Apr 16;51(4):875–880. doi: 10.1016/0006-291x(73)90008-9. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Clements J. E., Narayan O. cis- and trans-acting transcriptional regulation of visna virus. Science. 1985 Aug 2;229(4712):482–485. doi: 10.1126/science.2990051. [DOI] [PubMed] [Google Scholar]
- Kovacs E. J., Kelley J. Lymphokine regulation of macrophage-derived growth factor secretion following pulmonary injury. Am J Pathol. 1985 Nov;121(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Lairmore M. D., Rosadio R. H., DeMartini J. C. Ovine lentivirus lymphoid interstitial pneumonia. Rapid induction in neonatal lambs. Am J Pathol. 1986 Oct;125(1):173–181. [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Quérat G., Barban V., Sauze N., Filippi P., Vigne R., Russo P., Vitu C. Highly lytic and persistent lentiviruses naturally present in sheep with progressive pneumonia are genetically distinct. J Virol. 1984 Nov;52(2):672–679. doi: 10.1128/jvi.52.2.672-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J. A. GROWTH OF VIRULENT AND ATTENUATED ECTROMELIA VIRUS IN CULTURED MACROPHAGES FROM NORMAL AND ECTROMELIAIMMUNE MICE. J Immunol. 1964 Jun;92:837–842. [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Sihvonen L., Estola T., Tuomi J. Experimental maedi infection in sheep. 1. Detection of virus, clinical course, histopathology. Acta Vet Scand. 1980;21(1):113–123. doi: 10.1186/BF03546906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Woodward J. G., Frelinger J. A. Macrophage antiviral activity: extrinsic versus intrinsic activity. Infect Immun. 1982 May;36(2):672–677. doi: 10.1128/iai.36.2.672-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowring L., Haase A. T., Petursson G., Georgsson G., Palsson P., Lutley R., Roos R., Szuchet S. Detection of visna virus antigens and RNA in glial cells in foci of demyelination. Virology. 1985 Mar;141(2):311–318. doi: 10.1016/0042-6822(85)90264-8. [DOI] [PubMed] [Google Scholar]
- Trowbridge R. S., Lehmann J., Torchio C., Brophy P. Wild-type temperature-sensitive and -resistant visna viruses: isolation and biological comparison. Arch Virol. 1981;67(3):229–240. doi: 10.1007/BF01318133. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C. Correlation of persistent mouse hepatitis virus (MHV-3) infection with its effect on mouse macrophage cultures. Arch Virol. 1976;50(4):279–285. doi: 10.1007/BF01317953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by lymphokine-activated macrophages. Science. 1975 Jan 24;187(4173):261–263. doi: 10.1126/science.163038. [DOI] [PubMed] [Google Scholar]