Abstract
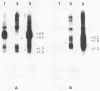
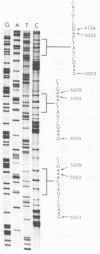
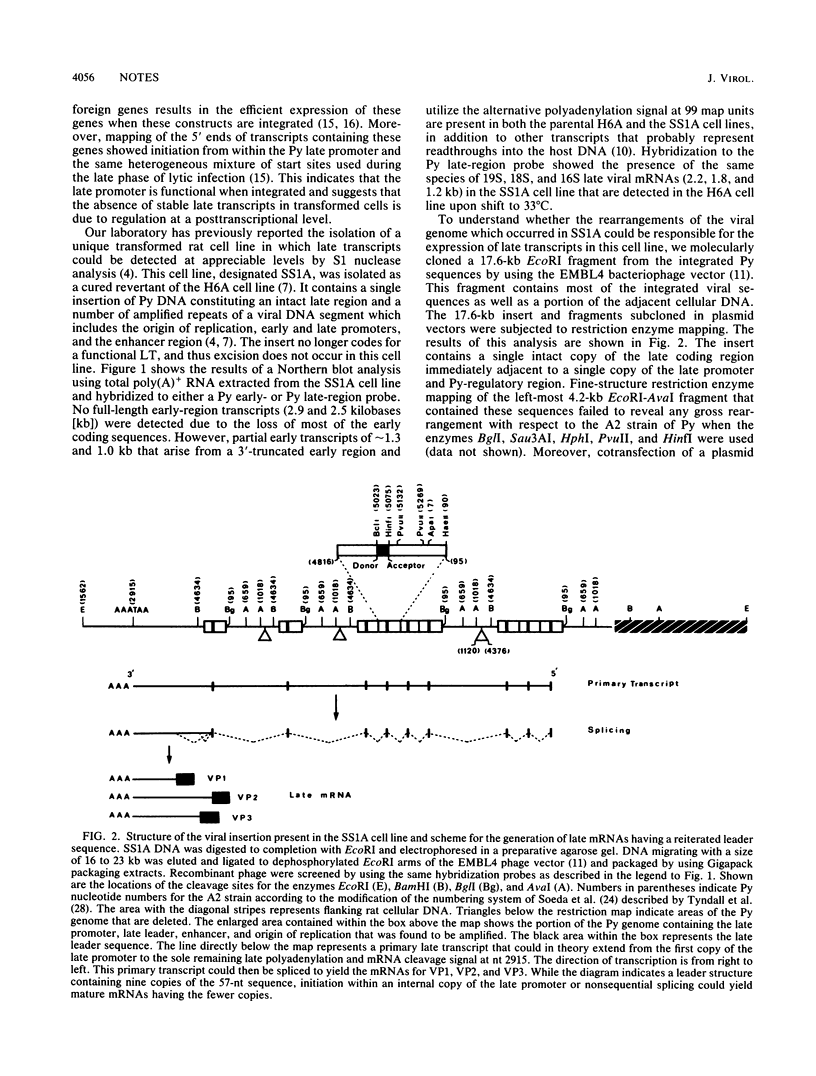
In cells transformed by polyomavirus, the viral genome is integrated into the host DNA, and in the absence of excision, viral gene expression is limited to the early region. We report here that the ability of a unique transformed rat cell line, designated SS1A, to produce readily detectable levels of late mRNAs is due to rearrangements of the integrated viral sequences. The structure of the SS1A insertion, resulting from amplification and deletion events, allows for the formation of a primary late transcript that can subsequently be spliced to generate a reiterated leader attached to the body of the late mRNA coding sequences. The presence of transcripts containing such a leader was confirmed by sequencing the 5' end of cDNA copies of late mRNAs isolated from a library constructed with SS1A mRNA. These results suggest that a reiterated leader sequence is necessary to stabilize late mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H. Kinetics and efficiency of polyadenylation of late polyomavirus nuclear RNA: generation of oligomeric polyadenylated RNAs and their processing into mRNA. Mol Cell Biol. 1984 Apr;4(4):722–729. doi: 10.1128/mcb.4.4.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson N. H. Polyoma virus giant RNAs contain tandem repeats of the nucleotide sequence of the entire viral genome. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4754–4758. doi: 10.1073/pnas.75.10.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami G. R., Carmichael G. G. Polyomavirus late leader region serves an essential spacer function necessary for viability and late gene expression. J Virol. 1986 May;58(2):417–425. doi: 10.1128/jvi.58.2.417-425.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Gattoni S., Zouzias D., Valle G. D. Loss of integrated viral DNA sequences in polyomatransformed cells is associated with an active viral A function. Cell. 1979 Jul;17(3):645–659. doi: 10.1016/0092-8674(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Basilico C., Zouzias D., Della-Valle G., Gattoni S., Colantuoni V., Fenton R., Dailey L. Integration and excision of polyoma virus genomes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):611–620. doi: 10.1101/sqb.1980.044.01.064. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Dailey L., Basilico C. Amplification of integrated viral DNA sequences in polyoma virus-transformed cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3850–3854. doi: 10.1073/pnas.77.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Cowie A., Tyndall C., Kamen R. Sequences at the capped 5'-ends of polyoma virus late region mRNAs: an example of extreme terminal heterogeneity. Nucleic Acids Res. 1981 Dec 11;9(23):6305–6322. doi: 10.1093/nar/9.23.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton R. G., Basilico C. Viral gene expression in polyoma virus-transformed rat cells and their cured revertants. J Virol. 1981 Oct;40(1):150–163. doi: 10.1128/jvi.40.1.150-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Kamen R., Favaloro J., Parker J. Topography of the three late mRNA's of polyoma virus which encode the virion proteins. J Virol. 1980 Feb;33(2):637–651. doi: 10.1128/jvi.33.2.637-651.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F. G., Basilico C. Transcription from the polyoma late promoter in cells stably transformed by chimeric plasmids. Mol Cell Biol. 1985 Apr;5(4):797–807. doi: 10.1128/mcb.5.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F. G., Pellegrini S., Cowie A., Basilico C. Regulation of polyomavirus late promoter activity by viral early proteins. J Virol. 1986 Oct;60(1):275–285. doi: 10.1128/jvi.60.1.275-285.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Firak T. A., Schroll C. T., Subramanian K. N. Activation of gene expression is adversely affected at high multiplicities of linked simian virus 40 enhancer. Proc Natl Acad Sci U S A. 1986 May;83(10):3199–3203. doi: 10.1073/pnas.83.10.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix J., Tseng R. W., Acheson N. H. Duplication of functional polyadenylation signals in polyomavirus DNA does not alter efficiency of polyadenylation or transcription termination. J Virol. 1986 Jun;58(3):733–742. doi: 10.1128/jvi.58.3.733-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Flavell A. J., Cowie A., Kamen R. Amplification in the leader sequence of late polyoma virus mRNAs. Cell. 1979 Feb;16(2):373–388. doi: 10.1016/0092-8674(79)90013-8. [DOI] [PubMed] [Google Scholar]
- Legon S. The binding of ribosomes to polyoma virus RNA. Possible role of the leader region in initiation site recognition. J Mol Biol. 1979 Oct 25;134(2):219–240. doi: 10.1016/0022-2836(79)90033-0. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., Dailey L., Basilico C. Amplification and excision of integrated polyoma DNA sequences require a functional origin of replication. Cell. 1984 Apr;36(4):943–949. doi: 10.1016/0092-8674(84)90044-8. [DOI] [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Sequence repeats in a polyoma virus DNA region important for gene expression. J Virol. 1983 Jul;47(1):233–237. doi: 10.1128/jvi.47.1.233-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Treisman R. Characterisation of polyoma late mRNA leader sequences by molecular cloning and DNA sequence analysis. Nucleic Acids Res. 1980 Nov 11;8(21):4867–4888. doi: 10.1093/nar/8.21.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng R. W., Acheson N. H. Use of a novel S1 nuclease RNA-mapping technique to measure efficiency of transcription termination on polyomavirus DNA. Mol Cell Biol. 1986 May;6(5):1624–1632. doi: 10.1128/mcb.6.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]