Abstract
BACKGROUND
Cardiovascular disease is the leading cause of mortality in patients with renal failure, accounting for more than 50% of deaths in end-stage renal disease. Risk factor modification with the use of cardioprotective medications such as angiotensin-converting enzyme inhibitors (ACEIs), beta-adrenergic antagonists (beta-blockers), acetylsalisylic acid (ASA) and 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors (statins) has been shown to reduce mortality in the general population.
OBJECTIVE
To determine the extent of use of these medications in a hemodialysis population.
METHODS
This was a cross-sectional study of a cohort of 185 prevalent hemodialysis patients. The inclusion criterion was dialysis dependence and there were no exclusion criteria. Data collection was by chart review. Contraindications to individual medication classes were not obtained.
RESULTS
There were 185 patients enrolled, the mean age was 63.42±15.1 years and 126 (68.1%) were male. Sixty-six (35.7%) patients had diabetes and 89 (48.1%) patients had established coronary artery disease (CAD). Forty-six (24.9%) patients were on ACEIs or angiotensin II receptor blockers, 59 (31.9%) were on beta-blockers, 70 (37.8%) were on ASA and 84 (45.4%) were on statins. Although these medications were used in fewer than 60% of patients, those with CAD were more likely to be prescribed an ACEI or an angiotensin II receptor blocker (P=0.026), a beta-blocker (P<0.001), ASA (P<0.001) or a statin (P=0.001) than those without CAD. There were no differences in the use of these medications between diabetic and nondiabetic patients.
CONCLUSIONS
Many hemodialysis patients are not prescribed cardioprotective medications. Given the high cardiovascular mortality in this high-risk population, more attention to reducing cardiovascular risk is warranted.
Keywords: Cardiorenal protection, Cardiovascular disease, Diabetes, Hemodialysis
Abstract
HISTORIQUE
Les maladies cardiovasculaires sont la principale cause de mortalité chez les patients atteints d’insuffisance rénale, représentant plus de 50 % des décès en cas d’insuffisance rénale terminale. Il est démontré que la modification des facteurs de risque grâce à des médicaments cardioprotecteurs comme des inhibiteurs de l’enzyme de conversion de l’angiotensine (IECA) des antagonistes béta-adrénergiques (béta-bloquants), de l’acide acétylsalicylique (ASA) et des inhibiteurs de la 3-hydroxy-3-méthylglutaryl coenzyme A réductase (statines) réduit le taux de mortalité dans la population générale.
OBJECTIF
Déterminer la portée de l’utilisation de ces médicaments au sein de la population des hémodialysés.
MÉTHODOLOGIE
Il s’agissait de l’étude transversale d’une cohorte de 185 hémodialysés prévalents. Les critères d’inclusion étaient la dépendance à la dialyse. Il n’y avait pas de critères d’exclusion. La collecte de données s’est effectuée par analyse des dossiers. On n’a pas obtenu les contre-indications aux classes de médicaments.
RÉSULTATS
Cent quatre-vingt-cinq patients ont participé, d’un âge moyen de 63,42±15,1 ans, dont 126 (68,1 %) étaient de sexe masculin. Soixante-six (35,7 %) étaient diabétiques et 89 (48,1 %) souffraient d’une coronaropathie établie. Quarante-six patients (24,9 %) prenaient des IECA ou des antagonistes de l’angiotensine II, 59 (31,9 %), des béta-bloquants, 70 (37,8 %), de l’ASA et 84 (45,4 %), des statines. Même si moins de 60 % des patients prenaient ces médicaments, ceux atteints d’une coronaropathie étaient plus susceptibles d’avoir une prescription d’IECA ou d’antagonistes de l’angiotensine II (P=0,026), de béta-bloquants (P<0,001), d’ASA (P<0,001) ou de statines (P=0,001) que ceux qui n’avaient pas de coronaropathie. Il n’y avait pas de différence d’usage de ces médicaments chez les diabétiques et les non-diabétiques.
CONCLUSIONS
De nombreux hémodialysés n’ont pas de prescription de médicaments cardioprotecteurs. Étant donné le taux élevé de mortalité cardiovasculaire au sein de cette population très vulnérable, il faut accorder plus d’attention à la réduction du risque cardiovasculaire.
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with stage 5 chronic kidney disease (CKD 5), accounting for more than 50% of deaths. After adjusting for age, race, sex and diabetes mellitus, CVD mortality in CKD 5 remains 10 to 20 times higher than in the general population (1). CVD in patients with CKD 5 is multifactorial. In addition to the higher prevalence of traditional cardiac risk factors (2), there is also the contribution of nontraditional cardiac risk factors unique to uremia, including anemia, abnormal calcium-phosphate homeostasis, inflammation, hyperhomocysteinemia, hypervolemia, dialysis dose and modality (3).
In the general population, large-scale trials in high-risk individuals have confirmed the value of primary prevention of CVD events with angiotensin-converting enzyme (ACE) inhibition (4), lipid-lowering therapy (5) and acetylsalicylic acid (ASA) (6). These benefits have not been realized in the CKD population, either because many studies have excluded patients with CKD or because these secondary intervention strategies are simply poorly applied in this population.
The purpose of the present study was to describe the use of four types of cardioprotective medications (ACE inhibitors [ACEIs], statins, ASA and beta-blockers) in a large cohort of patients receiving hemodialysis in a tertiary care centre. Although overall CVD risk is high simply by virtue of having end-stage renal disease (ESRD), we separately examined patients with diabetes and those with an established history of coronary artery disease (CAD).
PATIENTS AND METHODS
Study subjects
This was a cross-sectional study performed at a single academic tertiary care centre in a prevalent hemodialysis population. Participants were enrolled from the Kingston General Hospital dialysis unit (Kingston, Ontario) and affiliated satellite units between August 2002 and December 2002 if they had been receiving hemodialysis treatments three times weekly for a minimum of three months. No intervention was involved. There were no exclusion criteria, and written consent was obtained from all patients enrolled in the study. The study protocol was approved by the Queen’s University and Affiliated Teaching Hospitals Health Sciences Human Research Ethics Board.
Data collection
Baseline demographic and clinical data for all patients were obtained from hospital and clinical records. Demographic data included age, sex and the date of initiation of renal replacement therapy. Clinical data included etiology of renal failure, presence of diabetes mellitus and presence of CAD. Diabetic status was defined by a current use of oral hypoglycemic agents or insulin and/or a history of diabetes mellitus. A patient was categorized as having CAD if there was a history of angina or myocardial infarction (MI) and/or objective evidence (electrocardiogram, echocar-diogram, stress test, angiography/angioplasty, coronary artery bypass grafting or other cardiac imaging) to support the diagnosis. Biochemical data were obtained from monthly bloodwork collected over the previous year. Levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol and triglycerides, measured every two months over the previous year, were recorded. Average values for each variable were used for analysis. Hyperlipidemia was defined by an LDL-C level of greater than 2.6 mmol/L or the current use of a statin.
Medication use details for December 2002 were obtained and patient prescriptions for ASA, statins, ACEIs, angiotensin II receptor blockers (ARBs), beta-blockers or calcium channel blockers were recorded. Antihypertensive agents classified as ‘other’ included alpha-blockers and centrally acting agents. ACEIs and ARBs were classified together as ACEI/ARB. Nitrate use was not recorded. Contraindications to the individual drug classes were not obtained.
Statistical methods
All data were analyzed using SPSS (Version 12.0, SPSS Inc, USA). Descriptive statistics (means and standard deviations for continuous data, frequencies for categorical values) were generated for all variables. Bivariate analyses included Student’s t test and χ2 tests (Pearson’s or Fisher’s exact test, where appropriate). All statistical tests were two-sided, and P≤0.05 was considered statistically significant.
RESULTS
One hundred eighty-five subjects were enrolled in the study. The baseline characteristics of the study population are shown in Table 1. Mean age was 63.42±15.1 years and 126 (68.1%) of all patients were men. Sixty-six (35.7%) patients had diabetes, and 89 (48.1%) had established CAD. Thirty-eight (20.5%) patients had both diabetes and established CAD. Hyperlipidemia was present in 86 (46.5%) patients.
TABLE 1.
Demographics and clinical features of the study population
| Variable | All patients |
|---|---|
| Age, years (mean ± SD) | 63.42±15.1 |
| Male sex, n (%) | 126 (68.1) |
| Etiology of renal failure, n (%) | |
| Diabetes | 66 (35.7) |
| Renovascular | 44 (23.8) |
| Glomerulonephritis | 30 (16.2) |
| Reflux/obstructive | 12 (6.5) |
| Polycystic kidney disease | 10 (5.4) |
| Other | 23 (12.4) |
| Coronary artery disease, n (%) | 89 (48.1) |
| Diabetes mellitus, n (%) | 66 (35.7) |
| Total cholesterol, mmol/L | 4.30±0.90 |
| Low-density lipoprotein cholesterol, mmol/L | 2.19±0.70 |
| High-density lipoprotein cholesterol, mmol/L | 1.13±0.32 |
| Triglycerides, mmol/L | 2.20±1.76 |
| Number of antihypertensives, n (%) | |
| None | 60 (32.4) |
| One | 69 (37.3) |
| Two | 37 (20.0) |
| Three | 17 (9.2) |
| Four | 2 (1.1) |
Table 2 shows cardioprotective medication prescription by risk category. In the cohort as a whole, only 46 (24.9%) were prescribed an ACEI/ARB, 59 (31.9%) a beta-blocker, 70 (37.8%) ASA, and 84 (45.4%) a statin. No patient was prescribed an ACEI and an ARB simultaneously. Of patients with established CAD, 29 (32.6%) were taking an ACEI/ARB, 40 (44.9%) a beta-blocker, 49 (56.3%) ASA and 52 (61.2%) a statin. The number of patients prescribed none, one, two, three or all four classes of cardioprotective medications were 25%, 31.7%, 23.9%, 13.9% and 5.6%, respectively. These figures did not differ significantly among subgroups (Table 3). Twenty-five per cent of the cohort was taking warfarin, and there was no association between the use of warfarin and the prescription of ASA (data not shown).
TABLE 2.
Cardioprotective medication use and low-density lipoprotein cholesterol (LDL-C) level by risk category in hemodialysis patients*
| Risk category | ACEI/ARB | Beta- blocker | ASA | Statin | LDL-C >2.5 mmol/L |
|---|---|---|---|---|---|
| Cohort (n=185) | 24.9 | 31.9 | 37.8 | 45.4 | 29.2 |
| DM (n=66) | 27.3 | 29.7 | 39.1 | 51.6 | 35.1 |
| CAD (n=89) | 32.6 | 44.9 | 56.3 | 59.1 | 32.6 |
| CAD+/DM+ (n=38) | 31.6 | 39.5 | 50.0 | 52.6 | 40.5 |
| CAD+/DM− (n=51) | 33.3 | 49.0 | 61.2 | 64.0 | 26.5 |
Expressed as percentage. ACEI/ARB Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; ASA Acetylsalicylic acid; CAD Coronary artery disease; CAD+ Established history of CAD; DM Diabetes mellitus; DM+ DM present; DM− DM absent
TABLE 3.
Absolute number of cardioprotective agents used in a hemodialysis population according to risk category*
| Risk category | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Cohort (n=185) | 25.0 | 31.7 | 23.9 | 13.9 | 5.6 |
| DM (n=66) | 25.0 | 29.7 | 23.4 | 17.2 | 4.7 |
| CAD (n=89) | 11.5 | 23.0 | 34.5 | 20.7 | 10.3 |
| CAD+/DM+ (n=38) | 13.2 | 28.9 | 34.2 | 18.4 | 5.3 |
| CAD+/DM− (n=51) | 10.2 | 18.4 | 34.7 | 22.4 | 14.3 |
Expressed as percentage. CAD Coronary artery disease; CAD+ Established history of CAD; DM Diabetes mellitus; DM+ DM present; DM− DM absent
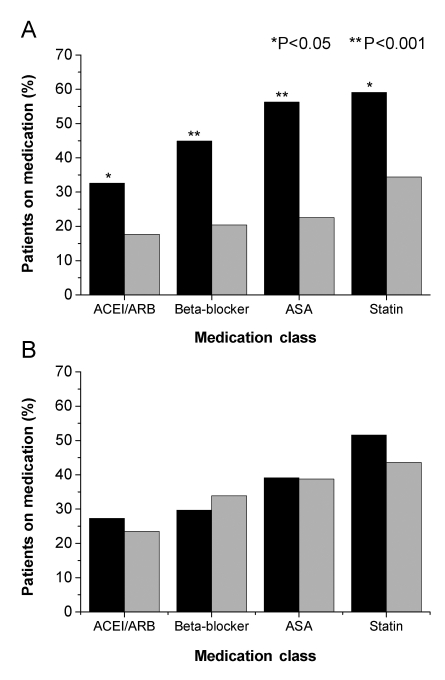
χ2 testing within the entire cohort showed that patients with a history of CAD were more likely to be on an ACEI/ARB (P=0.026), a beta-blocker (P<0.001), ASA (P<0.001) or a statin (P=0.001) than those without CAD (Figure 1A). There was no statistically significant association between diabetic status and the use of any cardioprotective medications (Figure 1B). There was no statistically significant association between dialysis vintage and the prescription of any cardioprotective medications (Table 4).
Figure 1.
A Cardioprotective medication use in hemodialysis patients with and without coronary artery disease. Significantly more patients with coronary artery disease (black bars) received the cardioprotective medications angiotensin-converting enzyme inhibtors (ACEIs), angiotensin II reception blockers (ARBs), beta-blockers, acetylsalicylic acid (ASA) and statins than those without established coronary artery disease (light gray bars). B Cardioprotective medication use in hemodialysis patients with and without diabetes. There were no significant differences in the use of ACEI/ARB, beta-blockers, ASA and statins in those with diabetes (black bars) compared with those without diabetes (light gray bars)
TABLE 4.
Cardioprotective medication use according to dialysis vintage*
| Dialysis vintage | ACEI/ARB | Beta-blocker | ASA | Statin |
|---|---|---|---|---|
| <1 year | 15.6 | 40 | 43.3 | 53.3 |
| 1–3 years | 26.1 | 36.8 | 40.3 | 52.2 |
| 3–5 years | 27.0 | 24.3 | 40.5 | 37.8 |
| >5 years | 28.8 | 28.9 | 34.1 | 37.8 |
Expressed as percentage. ACEI/ARB Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; ASA Acetylsalicylic acid
After two years of follow-up, 48 patients had died. Thirteen patients were censored at transfer to peritoneal dialysis, transplantation or to another dialysis facility. Demographic factors associated with mortality included age (P<0.001) and the presence of CAD (P=0.004). There was a trend toward an association between lower albumin concentration and mortality (P=0.07). The only cardioprotective medication associated with increased mortality was ASA (P=0.004).
DISCUSSION
Few other studies report cardioprotective medication prescription patterns in stable chronic hemodialysis patients. We conducted a cross-sectional retrospective study to describe such prescription patterns in a single-centre hemodialysis population. Our study demonstrates that there is a low use of cardio-protective therapies in the hemodialysis population, despite their proven benefits in the general population.
ACEIs
There is compelling evidence in the general population that ACEIs reduce CVD events and mortality (4,7). There are no randomized controlled trials evaluating the role of ACEIs in the reduction of CVD events in the hemodialysis population. However, a large cross-sectional study of ESRD patients admitted with an MI between 1994 and 1996 demonstrated a similar reduction in 30-day mortality between the ESRD and non-ESRD groups that was attributable to an ACEIs (8). Efrati et al (9) alsoreported in a retrospective cohort study that ACEIs significantly reduced all-cause mortality in dialysis patients, reporting a risk reduction of 52% despite similar blood pressure control between groups.
In our entire cohort of 185 patients, only 24.9% were prescribed an ACEI/ARB. In the subgroups of patients with diabetes and those with CAD, only 27.3% and 32.6%, respectively, were taking an ACEI/ARB. A significantly higher number of patients with CAD were on ACEI/ARB compared with those without CAD, but these medications were still used in less than one-third of this population. There were no differences in the use of ACEI/ARB between diabetics and nondiabetics (Figure 1B), despite strong evidence that these agents reduce cardiovascular mortality in the diabetic group (10–12).
The low prescription rate of ACEI/ARB may be partially attributable to safety concerns. Knoll et al (13) reported that the use of ACEIs and ARB was independently associated with an increased risk of developing hyperkalemia in dialysis patients. It is also conceivable that ACEI/ARB are discontinued in the late stages of CKD and are not restarted once a patient begins dialysis. We argue that if dialysis patients require antihypertensive medication to control blood pressure in addition to achievement of dry weight status, consideration for the use of an ACEI/ARB to encourage left ventricular hypertrophy (LVH) regression and for overall CVD benefit should be made. Dialysis patients on these agents will require close monitoring of their serum potassium levels, but this should not be a contraindication to their prescription. The FOSInopril in DIALysis (FOSIDIAL) trial (14), which is currently in progress, is attempting to assess the efficacy and safety of fosinopril in reducing mortality and cardiovascular events in hemodialysis patients with LVH.
Beta-blockers
It is well established that beta-blockers are beneficial in the secondary prevention of CVD events in the general population (15,16). However, beta-blockers may be underprescribed in the CKD population post-MI (8,17). An analysis of a prospective coronary care unit registry comprising 1724 patients with ST-segment elevation MI demonstrated a graded decrement in long-term survival across increasing renal dysfunction strata, part of which appeared to be mediated through the lower use of mortality-reducing therapy (17). Similar results were reported by Berger et al (8), who showed that following an acute MI, hemodialysis patients were significantly less likely to be prescribed ASA, beta-blockers or ACEIs than non-ESRD patients. Such findings bring attention to an area where considerable improvement can be made.
Beta-blockers reduce mortality in patients with left ventricular systolic dysfunction (18). Notably, at dialysis inception, systolic dysfunction and dilated cardiomyopathy are present in 15% and 32% of patients, respectively (19). Cice et al (20) performed a prospective trial of 114 dialysis patients with dilated cardiomyopathy randomly assigned to either carvedilol or placebo and were followed for up to 24 months. The active treatment group showed smaller cavity diameters and higher ejection fractions over time. They also had significantly fewer CVD-related deaths and hospital admissions than the placebo group.
Overall, in our cohort of hemodialysis patients, 32% were taking a beta-blocker. Of those with established CAD, only 45% were taking a beta-blocker. It is not clear why there may be a bias toward nonprescription of beta-blockers in the ESRD population, although it may be related to potential adverse effects. Beta-blockers are known to aggravate certain conditions, such as peripheral vascular disease and impotence, for which ESRD patients are at considerable risk. But the prevalence of these potential side effects in this population is unknown and has not been studied. Concerns about latent left ventricular dysfunction or the inability to mount a cardiac response to hypotension during dialysis treatments are other possible reasons.
ASA
Long-term ASA therapy confers benefit on risk of subsequent MI, stroke or vascular death among patients with CVD (21). There are few data, however, to support or detract from the use of ASA in dialysis patients. In a collaborative meta-analysis of randomized trials of antiplatelet therapy (22), which included 14 studies composed of 2632 dialysis patients, antiplatelet therapy produced a 41% proportional reduction in serious vascular events. However, the bleeding hazard could not reliably be determined from these data.
A National Kidney Foundation panel recommended the use of ASA (75 mg/day to 325 mg/day) for secondary prevention in dialysis patients (23). In spite of this, only 37% of our cohort was taking ASA. Those with established CAD were more likely to be taking ASA than those without CAD (P<0.001); however, it was prescribed to only 56.3% of these patients. There was no significant difference in the use of ASA in diabetics compared with nondiabetics, irrespective of history of CAD.
There are a number of reasons why ASA may not be prescribed to dialysis patients. A legitimate concern is the overall higher bleeding risk due to the inherent platelet dysfunction that occurs in uremia. Oral anticoagulant use may also be considered another relative contraindication. However, this study did not demonstrate any association between the use of war-farin and the non-use of ASA, and therefore does not support the argument that ASA may be avoided due to concomitant use of warfarin. Other contraindications to ASA use, such as previous hemorrhage, may be a factor. No randomized controlled trials have been done in this population to show benefit without added risk.
Statins
The prevalence of dyslipidemias in dialysis patients is high. According to definitions from the Adult Treatment Panel III (24), only 20.2% of dialysis patients have normal lipid levels. There is an inverse relationship between cholesterol levels and mortality in the ESRD population, likely due to the cholesterol-lowering effect of systemic inflammation and malnutrition (25). In the absence of inflammation, data suggest that an elevated serum cholesterol level is an independent risk factor for all-cause and CVD mortality (26).
In the general population, lowering serum cholesterol has resulted in reduced mortality, reduced ischemic events and reduced need for revascularization procedures in both primary (5) and secondary prevention (27,28). Statins successfully reduce LDL-C levels in patients with CKD, but are infrequently used in CKD (29). Analysis of the United States Renal Data System Dialysis Morbidity and Mortality Study (Wave 2) study data showed that only 9.7% of patients were taking a statin, but that its use was independently associated with a reduced risk of total mortality as well as CVD-specific mortality (30). A Canadian study reported that only 29.4% of dialysis patients with established CVD were taking lipid-lowering drugs (31), and only 16% of the Choices for Healthy Outcomes in Caring for ESRD study (CHOICE) cohort were using a lipid-lowering agent (2).
Patients with CKD are at increased risk for CVD; hence, it might be expected that lipid-lowering therapy would have a significant impact on vascular events in this population. However, whether cholesterol reduction translates into morbidity or mortality benefit in CKD remains controversial. The Medical Research Council/British Heart Foundation Heart Protection Study (28) showed that those patients with very early CKD had similar CAD benefit from statin therapy as those with normal renal function, showing a 25% risk reduction for vascular events. In contrast, recent evidence suggests no benefit of lipid-lowering agents in more advanced stages of CKD. The recently published Deutsche Diabetes Dialyse Studie (4D) trial (32) was a multicentre, randomized, double-blind prospective study of 1255 hemodialysis patients with type 2 diabetes mellitus who were randomly assigned to receive 20 mg/day of atorvastatin or matching placebo. This study demonstrated no benefit of atorvastatin in preventing the primary composite end point of death from cardiac causes, nonfatal MI and stroke. A second large-scale prospective study, the Study of Heart and Renal Protection (SHARP), is currently underway (33). This is a randomized controlled trial designed to study the effect of lipid-lowering therapy on major vascular events in CKD, both in predialysis and dialysis patients.
In our entire cohort, 47% of patients were taking a statin. Seventy per cent achieved the Kidney Disease Outcomes Quality Initiative (K-DOQI) target of LDL-C less than 2.6 mmol/L, with just 48% of them requiring a statin to reach the target. Of the 53% not taking a statin, nearly one-third had LDC-C levels above 2.6 mmol/L (data not shown). In the subgroup with CAD, 59.1% were on statins, but 32.6% of patients continued to have LDL-C levels above the recommended target. Similarly, 51.6% of diabetic patients were on statins, but 35.1% remained above target. Our patient population had a higher statin use than those in previous reports, but overall, 30% were not reaching the target either through failure to take the statin or undertreatment.
Low prescription rates in the dialysis population have historically been difficult to explain, but enthusiasm for lowering cholesterol may have been dampened by the reports associating hypocholesterolemia with mortality (25). In contrast, several small short-term trials (29,34) of statins in patients with ESRD have shown that statins were effective in lowering LDL-C levels and could safely be used in this population. Until the publication of the 4D trial, conclusive data regarding the use of lipid-lowering therapy were unavailable. A task force assembled in 1998 had sought to determine appropriate clinical practice guidelines (CPGs) for managing dyslipidemias in CKD (10). The task force recommended statin therapy if the target LDL-C level of less than 2.59 mmol/L was not reached despite three months of dietary intervention. This may, in part, explain the increased use of statins compared with the other classes of drugs in this cohort. The unexpected results of 4D trial demonstrate that one cannot rely on observational studies that are uncontrolled, and suggest the presence of additional pathogenic factors in the development of cardiovascular disease in uremic patients. A review of the 4D trial, however, indicates a lower cardiovascular event rate than expected, suggesting that the study may have been underpowered. It therefore remains prudent, until further data are available, to consider that all dialysis patients have high CVD risk and should thus be treated to the recommended target LDL-C level of less than 2.6 mmol/L.
Mortality
After two years of follow-up, 48 (28.1%) patients had died. Age and CAD were highly associated with mortality. There was a trend toward an association between mortality and lower albumin concentration levels. There was no association in this cross-sectional analysis between the use of ACEI/ARB, beta-blockers or statins, and mortality. However, the use of these medications was determined at a single point in time, and the study was not powered to examine the association between the use of individual drug classes and mortality. Previous cross-sectional studies (29,30) have demonstrated an association between the use of lipid-lowering agents and lower mortality in dialysis patients. However, this result was not duplicated in a single published randomized controlled trial to date. The prescription of ASA was significantly associated with mortality. Patients with CAD were more likely to be prescribed ASA (P<0.001), and therefore, this likely represents a subgroup at higher risk.
CONCLUSIONS
The results of the present study suggest that secondary prevention strategies are not widely adhered to in dialysis patients. Each class of medications was prescribed to less than one-half of the entire cohort. The subgroup with documented CAD was most likely to be prescribed these medications; however, they were still used in less than two-thirds of those patients. Treatment in the diabetic population seemed particularly problematic. The Canadian Diabetes Association CPGs recommend the use of ACEIs, antiplatelet therapy and lipid control for vascular protection (35). Unless contraindicated, all diabetic patients should be on low-dose ASA therapy (80 mg/day to 325 mg/day) and those who have LDL-C levels higher than 2.5 mmol/L should be on a statin. Our findings suggest that CVD risk factor management in our diabetic dialysis population is especially suboptimal.
There are many potential reasons for the non-use of cardio-protective medications in dialysis patients. Concerns have been raised regarding the potential adverse effects of these drugs and the lack of randomized controlled trials showing benefit in this population. Patients may already be on a large number of medications, so the issues of polypharmacy and noncompliance are potential deterrents to prescription of more medications. It is also plausible that there is therapeutic nihilism toward risk reduction, given the high burden of CVD and comorbidities in this population. Furthermore, the patho-physiology of CVD in CKD is still not well understood, raising skepticism about the efficacy of therapies aimed at traditional risk factor reduction. Patients with CKD do have a higher burden of atherosclerotic CAD compared with age- and sex-matched individuals with normal renal function, with higher prevalence of cardiovascular calcification. But there are other multiple additive factors, including hemodynamic overload, LVH and multiple metabolic abnormalities that occur in the setting of uremia, that likely also play a role in the pathogenesis of CVD in this group.
Whatever the reasons, the underuse of secondary prevention strategies represents a focus for improvement in quality of care. A noteworthy observation, however, is that the area of secondary prevention most adhered to was that of lipid management. It is conceivable, then, that the development and publication of CPGs has managed to successfully influence prescription practice in this area.
There are limitations of the present study. It was performed in a single nephrology centre and may not reflect the cardiovascular medication prescribing patterns of all Canadian nephrologists. Furthermore, contraindications to the use of different classes of cardiovascular medications in individual patients were not sought and patient adherence was not addressed. It was not determined whether any of these medications were previously used and later discontinued. Dialysis care in the Kingston General Hospital is provided by eight nephrologists. Patient care is reviewed a minimum of once weekly, and physicians tend to the dialysis unit daily.
The results of the present study suggest that nephrologists in our centre may be reluctant to extrapolate results from the general population to patients who have ESRD. The degree to which similar prescription practices occur elsewhere has not been adequately studied. Whatever the cause of the low prescription of cardiovascular risk-reducing medications, it is clear that randomized controlled trials in CKD patients are required to determine whether therapeutic modification of traditional risk factors will reduce CVD morbidity and mortality in this population.
ACKNOWLEDGEMENTS
The authors thank Dr AR Morton (Queen’s University, Kingston, Ontario) for his comments and suggestions about the manuscript. A preliminary account of some of these results was presented at the 36th Annual Meeting of the American Society of Nephrology in San Diego, California.
REFERENCES
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol. 2002;13:1918–27. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 3.Madore F. Uremia-related metabolic cardiac risk factors in chronic kidney disease. Semin Dial. 2003;16:148–56. doi: 10.1046/j.1525-139x.2003.16031.x. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G Heart Outcomes Prevention Evaluation Study Investigators. Effects on angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 5.West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS) Circulation. 1998;97:1440–5. doi: 10.1161/01.cir.97.15.1440. [DOI] [PubMed] [Google Scholar]
- 6.Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: Safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart. 2001;85:265–71. doi: 10.1136/heart.85.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox KM EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA Study) Lancet. 2003;362:782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 8.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–8. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 9.Efrati S, Zaidenstein R, Dishy V, et al. ACE Inhibitors and survival of hemodialysis patients. Am J Kidney Dis. 2002;40:1023–9. doi: 10.1053/ajkd.2002.36340. [DOI] [PubMed] [Google Scholar]
- 10.The Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and the MICRO-HOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 11.Niskanen L, Hedner T, Hansson L, Lanke J, Niklason A CAPPP Study Group. Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/beta-blocker-based treatment regimen: A subanalysis of the Captopril Prevention Project. Diabetes Care. 2001;24:2091–6. doi: 10.2337/diacare.24.12.2091. [DOI] [PubMed] [Google Scholar]
- 12.Lindholm LH, Ibsen H, Dahlof B, et al. LIFE Study Group. Lancet. Vol. 359. 2002. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol; pp. 1004–10. [DOI] [PubMed] [Google Scholar]
- 13.Knoll GA, Sahgal A, Nair RC, Graham J, van Walraven C, Burns KD. Renin-angiotensin system blockade and the risk of hyperkalemia in chronic hemodialysis patients. Am J Med. 2002;112:110–4. doi: 10.1016/s0002-9343(01)01068-3. [DOI] [PubMed] [Google Scholar]
- 14.Zannad F, Kessler M, Grünfeld JP, Thuilliez C FOSInopril in DIALysis Investigators. FOSIDIAL: A randomised placebo controlled trial of the effects of fosinopril on cardiovascular morbidity and mortality in haemodialysis patients. Study design and baseline characteristics. Fundam Clin Pharmacol. 2002;16:353–60. doi: 10.1046/j.1472-8206.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- 15.Beta-blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–14. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 16.Beta-blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction. II. Morbidity results. JAMA. 1983;250:2814–9. doi: 10.1001/jama.1983.03340200048027. [DOI] [PubMed] [Google Scholar]
- 17.McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am Heart J. 2002;144:226–32. doi: 10.1067/mhj.2002.125513. [DOI] [PubMed] [Google Scholar]
- 18.CIBIS-II Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBIS-II): A randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 19.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–92. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 20.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–44. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 21.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomized trials of antiplatelet therapy, I: Prevention of death, myocardial infarction and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:235–46. [PMC free article] [PubMed] [Google Scholar]
- 22.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Beto JA, Coronado BE, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease. What do we know? What do we need to learn? Where do we go from here? Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 24.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 25.Iseki K, Yamazato M, Tozawa M, Takishita S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002;61:1887–93. doi: 10.1046/j.1523-1755.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA. 2004;291:451–9. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 27.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 28.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 29.Harris KP, Wheeler DC, Chong CC. Atorvastatin in CAPD Study Investigators. A placebo-controlled trial examining atorvastatin in dyslipidemic patients undergoing CAPD. Kidney Int. 2002;61:1469–74. doi: 10.1046/j.1523-1755.2002.00262.x. [DOI] [PubMed] [Google Scholar]
- 30.Seliger S, Weiss NS, Gillen DL, et al. HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002;61:297–304. doi: 10.1046/j.1523-1755.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- 31.Prichard S. Coronary artery disease in end-stage renal disease: Risk factors and treatment strategies. J Jpn Soc Dial Ther. 2000;33:159–64. [Google Scholar]
- 32.Wanner C, Krane V, Marz W, et al. German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 33.Baigent C, Landry M. Study of Heart and Renal Protection (SHARP) Kidney Int. 2003;63(Suppl 84):S207–10. doi: 10.1046/j.1523-1755.63.s84.4.x. [DOI] [PubMed] [Google Scholar]
- 34.van den Akker JM, Bredie SJ, Diepenveen SH, van Tits LJ, Stalenhoef AF, van Leusen R. Atorvastatin and simvastatin in patients on hemodialysis: Effect on lipoproteins, C-reactive protein and in vivo oxidized LDL. J Nephrol. 2003;16:238–44. [PubMed] [Google Scholar]
- 35.Harris SB, Lank CN. Recommendations from the Canadian Diabetes Association. 2003 guidelines for prevention and management of diabetes and related cardiovascular risk factors. Can Fam Physicians. 2004;50:425–33. [PMC free article] [PubMed] [Google Scholar]