Abstract
BACKGROUND
Lowering plasma lipid levels in patients in the months following hospital discharge for a myocardial infarction (MI) is clearly beneficial if recurrent cardiac events and mortality are to be prevented; traditionally, however, there has been a large gap between guidelines and levels achieved in routine practice.
OBJECTIVES AND METHODS
A randomized, open-label clinical trial was conducted to assess the impact of nurse-centred surveillance and treatment in achieving nationally recognized lipid targets in post-MI patients. This program had the following features: systematic telephone follow-up of patients discharged from the University of Sherbrooke (Sherbrooke, Quebec) after an MI; systematic lipid testing three months after discharge; close liaison with, and guidance of, patients’ primary care physicians to intervene on results of this test if targets were not obtained; and continued monitoring of patients until lipid profiles consistent with consensus targets were achieved. The impact of this approach was tested and compared with that of a control group that continued to be followed by a primary care physician for up to 18 months.
RESULTS
A total of 127 patients were randomly assigned into an intervention group (n = 64) or a control group (n = 63). The intervention group was followed by telephone for an average (±SD) of 4.4 ± 2.0 months post-MI. At this point, when intervention was optimized, the mean low-density lipoprotein cholesterol (LDL-C) level was 2.19 ± 0.65 mmol/L in the intervention group, and 87.3% of patients had LDL-C levels of less than 2.5 mmol/L. Patients from both experimental groups returned at 12 months and 18 months post-MI for a new blood lipid assessment. In total, 12.5% of patients in each group were lost to follow-up. At 12 months and 18 months, the mean LDL-C level was not different between the two groups, nor was there a significant difference in the proportion of patients achieving LDL-C levels of less than 2.5 mmol/L (51.6% in the intervention group and 65% in the control group at 18 months; P > 0.05). When the combined end point of an LDL-C level of less than 2.5 mmol/L, a triglyceride level of less than 2.0 mmol/L and a total cholesterol to high-density lipoprotein cholesterol ratio of less than 4.0 was considered, the proportion of patients achieving this composite at 18 months was low and not different between the two groups (23.4% in the intervention group and 38.3% in the control group; P > 0.05). Over 95% of patients in both groups were on a lipid-lowering medication, and more than 90% had complied with their medication regimen at 18 months.
CONCLUSIONS
This trial did not support the role of nurse-managers and a system of telephone-based contacts to ensure the continuity of care and aggressive intervention when considering cardiovascular risk factors in post-MI patients. This trial also re-emphasized the important remaining treatment gap in secondary prevention of coronary artery disease, particularly if composite lipid end points are to be targeted.
Keywords: Case management, Disease management, Dyslipidemia, Myocardial infarction, Nursing, Patient care team, Randomized controlled trials, Specialties
Abstract
HISTORIQUE
La diminution des taux de lipides plasmatiques chez les patients dans les mois suivant leur congé de l’hôpital après un infarctus du myocarde (IM) est clairement bénéfique pour prévenir une récurrence des événements cardiaques et la mortalité, mais d’ordinaire, il y a un énorme écart entre les lignes directrices et les résultats obtenus dans la pratique quotidienne.
OBJECTIFS ET MÉTHODOLOGIE
Nous avons mené un essai clinique ouvert aléatoire visant à évaluer les répercussions d’une surveillance par une infirmière spécialisée et d’un traitement pour atteindre les cibles reconnues nationalement chez des patients après un IM. Ce programme était doté des caractéristiques suivantes : suivi systématique par téléphone des patients ayant reçu leur congé du Centre hospitalier universitaire de Sherbrooke à Sherbrooke, au Québec, après un IM; bilans lipidiques systématiques trois mois après le congé; contacts étroits avec les médecins traitants des patients et orientation de ces médecins pour qu’ils interviennent si les résultats démontrent que les cibles ne sont pas atteintes; et surveillance continue des patients jusqu’à ce que les profils lipidiques respectent les normes consensuelles. Les répercussions de cette démarche ont été vérifiées et comparées à celles d’un groupe témoin qui a continué à être suivi par un médecin de première ligne pendant une période maximale de 18 mois.
RÉSULTATS
Au total, 127 patients ont été répartis de manière aléatoire entre un groupe d’intervention (n = 64) et un groupe témoin (n = 63). Le groupe d’intervention a été suivi par téléphone pendant une moyenne ± ÉT de 4,4 ± 2,0 mois après un IM. À ce moment-là, lorsque l’intervention était optimisée, le taux de cholestérol à lipoprotéines de basse densité (C-LDL) était de 2,19 ± 0,65 mmol/L au sein du groupe d’intervention, et 87,3 % des patients présentaient des taux de C-LDL inférieurs à 2,5 mmol/L. Les patients des deux groupes expérimentaux sont revenus 12 et 18 mois après l’IM pour subir une nouvelle évaluation lipidique. Au total, seulement 12,5 % des patients de chaque groupe ont été perdus au suivi. À 12 et 18 mois, le taux de C-LDL moyen ne différait pas entre les deux groupes, et on ne constatait pas de différence significative dans la proportion de patients atteignant des taux de C-LDL inférieurs à 2,5 mmol/L (51,6 % au sein du groupe d’intervention et 65 % au sein du groupe témoin après 18 mois, P > 0,05). La proportion de patients ayant atteint simultanément un taux de C-LDL inférieur à 2,5 mmol/L, un taux de triglycérides inférieur à 2,0 mmol/L et un ratio entre le cholestérol total et le cholestérol à lipoprotéines de haute densité (C-HDL) inférieur à 4,0 ne différait pas entre les deux groupes après 18 mois (23,4 % au sein du groupe d’intervention et 38,3 % au sein du groupe témoin, P > 0,05). Plus de 95 % des patients des deux groupes prenaient des hypocholestérolémiants, et plus de 90 % adhéraient à ce traitement au bout de 18 mois.
CONCLUSIONS
Cet essai ne soutient malheureusement pas la prise en charge par des infirmières dédiées et un système de contacts téléphoniques pour garantir la continuité des soins et des interventions énergiques. Cet essai fait de nouveau ressortir l’important écart de traitement dans la prévention secondaire des coronaropathies, notamment quand plusieurs cibles lipidiques doivent être obtenues en même temps.
There is currently little dispute that plasma lipid levels should be lowered aggressively in patients who have had a myocardial infarction (MI) (1,2). However, although aggressive lipid lowering is now a very well-established treatment, and has been proven to be cost-effective (3), it is generally believed that large gaps exists between what is recommended in consensus guidelines (4,5) and what is actually achieved in routine clinical practice (6–8). Much effort has been invested during the past decade in demonstrating that large and comprehensive cardiac rehabilitation centres improve risk factor management and outcomes (9–12). However, only a relatively small proportion of patients with established coronary artery disease (CAD) has access to these facilities (13,14), and the overall cost of these centres, while not prohibitive, is nonetheless an obstacle to their wider adoption.
In attempt to address these matters, our group has also been working on a unique, nurse-centred surveillance and treatment program aimed at closing the large gap between nationally recognized guidelines for lipid lowering and current actual practice in the secondary prevention of CAD. This program has previously been described elsewhere (15) and has the following features: systematic follow-up (by telephone or in person) of every patient discharged from the University of Sherbrooke (Sherbrooke, Quebec) after the diagnosis of MI; systematic lipid testing three months after discharge from the hospital in a stable condition; close link (through telephone and letter communication) with patients’ primary care physicians, making them aware of test results and targets to be achieved; supervision of primary care physicians in their interventions in these high-risk patients; and close re-evaluation of the patient until treatment results in a lipid profile consistent with consensus targets. Using this integrated approach, we had previously published (15) that more than 90% of our post-MI patients could be followed systematically, and that lipid profiles at discharge from this nurse-managed program could be brought to what was considered minimal coronary risk at the time of the initiation of this project. Although the initial results were encouraging, our approach had never been studied in the setting of a controlled environment.
The goal of the current project, therefore, was to test, in a randomized, controlled trial, whether a nurse-managed approach was more effective than conventional care for patient adherence to treatment and for control of the lipid profile up to 18 months post-MI. More specifically, we were interested in using stricter lipid targets than those used in the past and in evaluating the proportion of patients who would be able to maintain these goals on a longer-term basis.
PATIENTS AND METHODS
Patient population
One hundred twenty-seven patients admitted to the Centre hospitalier universitaire de Sherbrooke (Sherbrooke, Quebec) (‘the Centre’) for an MI between January 1, 2001, and September 1, 2002, were randomly assigned to the present trial. The Centre is the major acute care hospital in the Eastern Townships area and has approximately 600 inpatient beds in two separate hospitals (Fleurimont and Bowen) 4 km apart; it serves a regional population of approximately 300,000. The patient population is relatively confined to this well-defined region and, as a result, relies heavily on a network of local primary care physicians for follow-up. Only patients younger than 70 years of age were included in the present study, because at the time of inception of the program, only limited data were available on the benefits of aggressive lipid-lowering interventions in older patients. Over the entire period of recruitment to the present project, a total 334 patients were discharged from the Centre with a primary diagnosis of MI based on information provided in their discharge summary. Therefore, the present nonconsecutive study sample represents 38% of the entire population that was available. Reasons for not being enrolled in the present trial included age older than 70 years (17%), uncertain diagnosis of MI based on laboratory and electrocardiogram results (9.2%), coronary artery bypass grafting (6.9%) and associated severe concomitant complications preventing comparable follow-up (8.7%), refusal by the patient (12%) and hospitalization over a weekend or a holiday (8.2%).
Study design
The present study was a randomized, open-label trial in which patients intensively followed using a nurse-managed approach were compared with a group of patients followed by their regular physician (control group). The study was approved by the local Institutional Review Board and all patients signed an informed consent form. To ensure the appropriateness of the trial, the consent forms used for the two experimental groups were different. In the intensive group, patients were given detailed instructions on how they would be followed in this nurse-managed project; in the control group, details of the approach used in the intensive group were not revealed. Volunteers in the control group were asked only for permission to call their pharmacists and to return to the hospital at 12 months and 18 months post-MI for some blood tests, without specifically referring to lipid assessments.
The research nurse or dietician at the Centre met all the research volunteers before discharge from the hospital. Random assignment took place before meeting the potential volunteers because the informed consent form to be signed would depend on the group allocation. Even if the timing of the random assignment was slightly different from what is generally seen, we know a posteriori, from the number of patients that did not provide consent, that group allocation was not affected. On first contact with the patient, the relevant consent form was signed, and several demographic and medical variables were collected from the patient’s medical record. In the Centre, a complete lipid profile is routinely obtained within 24 h of admission for an MI, and the approach is intended not to obstruct the management of a patient in the acute period following MI – any decision to treat dyslipidemia in patients from both treatment groups during hospitalization was left to the cardiology team.
In the intervention group, and as previously described (15), each patient received a letter a few weeks after hospital discharge and was followed up with a telephone call, during which the coordinator reaffirmed the importance of obtaining a complete fasting lipid profile three months after the index MI. The call was also used to impart key educational messages on the importance of cholesterol management in the prevention of CAD, and the need for the long-term follow-up and treatment of this risk factor. Patients were able to attend either the Centre or one of the networks of regional hospitals or general practitioners to have their lipid profile determined. If a patient elected to attend a site other than the Centre, the results of the profile were forwarded by the hospital or the general practitioner to the Centre’s research team. This approach had previously been validated (15) in the Eastern Township area. The lipid goals to be obtained in patients randomly assigned to the intervention group were as follows: a low-density lipoprotein cholesterol (LDL-C) level of less than 2.5 mmol/L; a triglyceride (TG) level of less than 2.0 mmol/L; and a total cholesterol (TC) to high-density lipoprotein cholesterol (HDL-C) ratio of less than 4.0, as recommended in the Canadian guidelines (16) extant at the time of inception of the present protocol. If the three-month lipid profile showed values above these targets, the Centre’s research nurse-manager informed the patient and contacted the patient’s physician to recommend an appropriate intervention, namely, a dietary consultation (involving a dietician), and/or the prescription of a lipid-lowering medication or the adjustment of the patient’s existing medication. If the physician did not feel proficient enough to implement the intervention recommended, the patient was invited to attend the clinic at the Centre. In all other instances, the nurse-manager ensured that treatment had been proposed and initiated by contacting the patient two weeks after the physician was notified of the need for intervention.
Once the targets were reached, the volunteer was temporarily discharged from the intensive approach, but was recontacted at 12 months and 18 months after the index MI for a compliance assessment through the pharmacist, and a complete lipid profile was undertaken at the Centre.
In contrast to the measures adopted for the intervention group, no specific, short-term follow-up was performed in the control group. Decisions to measure and/or treat the lipid parameters were left entirely to the patients’ family physicians.
Primary end point
Efficacy assessment
The primary end point of the present study was the proportion of patients in each experimental group who registered the target LDL-C level of less than 2.5 mmol/L at 12 months and 18 months after hospital discharge. The TG and HDL-C level targets were examined as secondary outcomes in the context of an aggregate end point of simultaneously achieving an LDL-C level of less than 2.5 mmol/L, a TG level of less than 2.0 mmol/L and a TC:HDL-C ratio of less than 4.0, as recommended in the Canadian consensus guidelines at that time (16). With 56 completers per group (an attrition of approximately 12%), the study had 85% power to detect an absolute 25% difference on a theoretical baseline proportion of 60% of patients in the control group who would achieve an LDL-C level of less than 2.5 mmol/L (17). An absolute difference of 25% was considered clinically relevant and was determined prospectively. The sample size calculation was based on an alpha error of 0.05 in a two-sided test.
Other end points
Time spent with the nurse-manager
Time spent during each intervention by the nurse-manager over the phone or in person was recorded and used to evaluate the degree of intervention in both experimental groups. It is reported in total number of minutes over the 18-month follow-up.
Quality of life
The quality of life was examined in the two experimental groups at baseline (before the MI hospitalization discharge), and after 12 months and 18 months using the standard version of the Medical Outcomes Study 36-item Short Form health survey (SF-36) (18). Norm-based scores were used to assign all scales originally scored from 0 to 100 a mean of 50 and an SD of 10 in the general 1998 United States population. The hypothesis to be tested was that the intervention would have either a neutral or beneficial effect on the quality of life because patients would be better informed and less prone to anxiety. A scoring algorithm (QualityMetric Incorporated, USA) was used to produce scores on multi-item and summary scales, as well as to adjust scores for respondents in each experimental group who did not answer every survey item.
Persistence in the use of a lipid-lowering drug
Drug compliance was estimated in patients who were prescribed a lipid-lowering medication during the follow-up period. Compliance was measured using information gathered from the patient’s pharmacist, under informed consent, at 12 months and 18 months, and by obtaining a detailed history of each pharmacy visit made by the patient. A medication compliance index was then calculated, taking into account the number of tablets issued at a given visit, the date of the next visit to the pharmacy, as well as the number of days between the two visits. The compliance index, expressed in percentages and integrated over the entire period of follow-up, represented the proportion of days when a lipid-lowering medication was taken on schedule. This approach was previously validated by our group (15). Doses of lipid-lowering agents prescribed in both experimental groups were also calculated on these follow-up time points. Because many different agents were used in these patients, doses of lipid-lowering agents were expressed as a percentage of the maximum total daily dose recommended for a given agent. The following maximum daily doses were used for this calculation (in alphabetical order): atorvastatin (80 mg), bezafibrate (400 mg), cholestyramine (8 g), micronized fenofibrate (200 mg), gemfibrozil (1200 mg), pravastatin (40 mg), rosuvastatin (40 mg) and simvastatin (80 mg). For a fair comparison of the means of the two experimental groups, patients who were not on any lipid-lowering agent at 12 months and 18 months of follow-up were assigned a 0% value for this adjusted-dose end point.
Statistical analyses
Data are reported as means ± SDs. Comparisons between each group were performed using the Student’s t test for continuous variables. For nominal variables (eg, the proportion of patients reaching an LDL-C level of less than 2.5 mmol/L), the χ2 test was used. In some instances, when more than 20% of expected frequencies were less than five, a Fisher’s exact test was performed. A two-factor analysis of variance with interaction was also used to compare scores from the SF-36 across time and between treatment groups. P < 0.05 was considered statistically significant.
RESULTS
Patient baseline characteristics
Table 1 depicts the baseline characteristics of 127 patients randomly assigned to the intervention or the control groups in the present trial. The majority of subjects were male (approximately 80% to 85%), but there was no statistically significant difference between groups (P < 0.05). The mean age in each group was approximately 57 years. The proportions of smokers, and of patients with diabetes or at least one clinical CAD episode before their index events, were similar between the two experimental groups. As a whole, these patients were followed by 95 different general practitioners in the area. There is evidence that only 10 of these physicians (10.5%) followed a maximum of one or two patients in each experimental group over a period of up to 3.5 years.
TABLE 1.
Patient baseline characteristics
| Intervention group (n = 64) | Control group (n = 63) | P | |
|---|---|---|---|
| Age, years (mean ± SD) | 57.8 ± 9.6 | 56.9 ± 8.8 | NS* |
| Male sex, % | 89.1 | 77.8 | NS† |
| Currently smoking, % | 45.8 | 33.9 | NS† |
| Diabetes, % | 17.2 | 15.9 | NS† |
| Prior history of CAD, % | 41.3 | 28.6 | NS† |
Nonpaired Student’s t test;
χ2 test. CAD Coronary artery disease; NS Not significant
Lipid profiles during the acute phase of the index MI
A complete lipid profile was obtained during the first 24 h following an index MI in 89.1% of patients in the intervention group and 88.9% of patients in the control group, and the difference between these two proportions was not statistically different (P > 0.05).
Mean baseline values for each lipid parameter are shown in Table 2; there were no significant intergroup differences in any of these indexes. Reported use of lipid-lowering medications at the index hospitalization was also the same in the two groups (approximately 23%). Table 3 provides relevant information on the duration of hospitalization and the procedures performed during the index MI hospitalization in both groups. The similar proportions of all these variables in both groups provides assurance that the random assignment resulted in a homogeneous distribution of patients between the two experimental groups.
TABLE 2.
Lipid profile obtained in the 24 h following the index myocardial infarction
| Intervention group(n = 57) | Control group(n = 56) | P | |
|---|---|---|---|
| Plasma LDL-C, mmol/L (mean ± SD) | 2.80 ± 0.89 | 2.78 ± 1.04 | NS* |
| Plasma HDL-C, mmol/L (mean ± SD) | 0.94 ± 0.29 | 1.05 ± 0.31 | NS* |
| Plasma triglycerides, mmol/L (mean ± SD) | 1.99 ± 1.03 | 2.25 ± 1.36 | NS* |
| Plasma cholesterol, mmol/L (mean ± SD) | 4.61 ± 0.98 | 4.86 ± 1.15 | NS* |
| TC:HDL-C ratio (mean ± SD) | 4.99 ± 1.27 | 4.96 ± 1.78 | NS* |
| Patients on a lipid-lowering agent, % | 23.4 | 23.8 | NS† |
| Patients with LDL-C <2.5 mmol/L, % | 38.2 | 43.1 | NS† |
Nonpaired Student’s t test;
χ2 test. HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; TC Total cholesterol
TABLE 3.
Summary of in-hospital procedures and lipid therapy status at discharge
| Intervention Group (n = 64) | Control group (n = 63) | P | |
|---|---|---|---|
| Duration of hospitalization, days (mean ± SD) | 6.2 ± 3.4 | 7.4 ± 5.6 | NS* |
| Procedure performed during index admission, % | |||
| Thrombolysis | 23.4 | 22.2 | NS† |
| Stenting | 64.1 | 68.3 | NS† |
| PTCA | 6.3 | 4.8 | NS‡ |
| Initiation or modification of a lipid- lowering therapy before discharge, % | 71.9 | 66.7 | NS† |
Nonpaired Student’s t test;
χ2 test;
Fisher’s exact test (as frequencies of several cells were less than five). NS Not significant; PTCA Percutaneous transluminal coronary angioplasty
Time spent with nurse-manager in each experimental group
Over the course of the 18 months of follow-up, the nurse-manager spent an average (±SD) of 52.2 ± 29.8 min with each patient in the intervention group, compared with 19.3 ± 15.5 min in the control group (P < 0.001). This difference was entirely due to the fact that more time was spent with the intervention group patients in the three months following the index MI in an effort to convey a key educational message and to ensure that a lipid profile was drawn and an intervention was prescribed, if indicated. The time spent with the control group (mean of 19.3 min) was not of an interventional nature; instead, this time was used to retrieve the quality of life questionnaires and to ensure that the lipid profiles were performed at the 12- and 18-month follow-up time points. On average, patients randomly assigned to the intervention group were temporarily discharged from the nurse-led management program 4.4 ± 2.0 months after the index MI. At discharge, these patients had average plasma LDL-C levels of 2.23 ± 0.60 mmol/L, plasma HDL-C levels of 1.02 ± 0.21 mmol/L and plasma TG levels of 1.81 ± 0.87 mmol/L. Plasma LDL-C levels of less than 2.5 mmol/L were achieved in 87.3% of the patients, but only 42.9% met the lipid triple target recommended by the consensus guidelines.
Of the 127 patients enrolled in the present trial, 16 patients (12.6%) were lost during the 18-month follow-up; these were equally distributed between the two experimental groups (eight in the intervention group and eight in the control group). There were two deaths in the intervention group (one within one month post-MI and another one year after) and four in the control group (one six months post-MI and three at one year).
Lipid profiles at 12 months and 18 months after the index MI
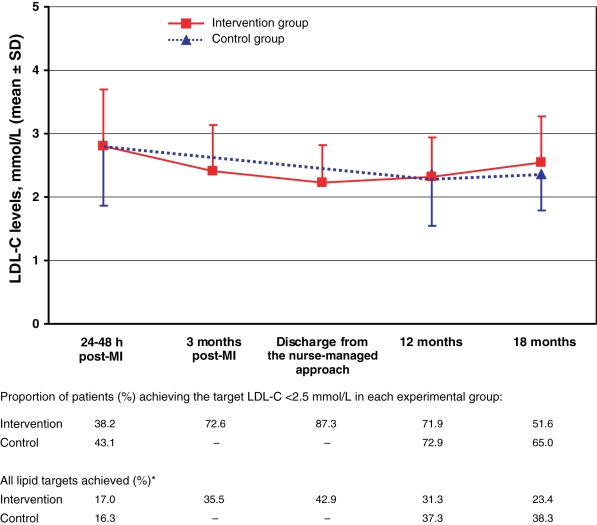
Lipid profiles were obtained for patients in both experimental groups 12 months and 18 months after the index MI. The same percentage of patients in the intervention group (87%) and the control group (86%) returned for their lipid profiles at 12 months and 18 months. Figure 1 shows changes in plasma LDL-C levels over time in both groups, and provides a detailed summary of the proportions of patients who achieved a plasma LDL-C level of less than 2.5 mmol/L or the composite lipid end point over time in each experimental group. There were no differences between the intervention and control groups over this 18-month follow-up period. More specifically, the 95% CIs for the difference in these proportions were −0.040 to 0.308 for reaching a plasma LDL-C level of less than 2.5 mmol/L and −0.013 to 0.311 for reaching all lipid targets at 18 months.
Figure 1.
Changes in plasma low-density lipoprotein cholesterol (LDL-C) levels and proportions of patients achieving various lipid targets over 18 months in the intervention (nurse-managed) and control groups. *Composite end point of an LDL-C level of less than 2.5 mmol/L, a triglyceride level of less than 2.0 mmol/L and a TC: high-density lipoprotein cholesterol ratio of less than 4.0. MI Myocardial infarction
Doses of lipid-lowering agents at 12 months and 18 months after the index MI
Because the usage and doses of lipid-lowering agents can influence the results outlined above, lipid-lowering treatment was examined at the 12-month and 18-month time points in both experimental groups. These results are depicted in Table 4. While there was a trend for the use of lipid-lowering agents at higher doses and in combination therapy in the intervention group, these differences were not statistically significant at 12 months or 18 months. Notably, all patients in the intervention group were on a lipid-lowering agent at the 12-month and 18-month follow-up time points, while 9% of patients in the control group were not receiving any of these agents (P < 0.03). In the intervention group, the apparent fall-off in LDL-C levels between discharge from the intervention and the 12-month time point does not appear to be related to doses of the lipid-lowering agents (45.8% ± 19.7% of maximum dose at discharge from nurse-led management versus 46.5% ± 20.6% of maximum dose at 12 months).
TABLE 4.
Status of lipid-lowering therapy at various time points during follow-up
| Follow-up at 12 months | Intervention group (n = 57) | Control group(n = 56) | P |
|---|---|---|---|
| Dose of lipid-lowering agent prescribed, % of maximal recommended dose (mean ± SD) | 46.5 ± 20.6 | 39.7 ± 26.3 | NS* |
| Number of patients without a lipid-lowering agent | 0 | 5 | <0.03† |
| Number of patients on a combination of lipid-lowering agents | 3 | 0 | NS† |
| Follow-up at 18 months | Intervention group (n = 56) | Control group (n = 55) | P |
| Dose of lipid-lowering agent prescribed, % of maximal recommended dose (mean ± SD) | 43.1 ± 23.0 | 37.0 ± 28.4 | NS* |
| Number of patients without a lipid-lowering agent | 0 | 5 | <0.03† |
| Number of patients on a combination of lipid-lowering agents | 3 | 0 | NS† |
See the ‘Patients and Methods’ section for details on calculating the percentage of maximal recommended dose;
Nonpaired Student’s t test;
Fisher’s exact test (as frequencies of several cells were less than five). NS Not significant
Persistence with lipid-lowering therapy
Compliance was consistently high in both experimental groups 12 months and 18 months after the index MI hospitalization, and there were no significant differences between them. At 12 months, 87.3% and 90.6% of the intervention and control groups, respectively, continued to take and renew their lipid-lowering medications on time. At 18 months, the persistence rate was 89.5% and 89.2% in the intervention and the control groups, respectively. At 18 months, 98.2% of patients in the intervention group and 90.9% of patients in the control group were on a lipid-lowering medication; more than 90% of prescriptions for lipid-lowering drugs were for 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins).
Quality of life assessment
Figure 2 summarizes the scores for the eight domains of the SF-36 and for the two summary scales (physical component and mental component) in each experimental group at three different time points during the study: at discharge from the index MI hospitalization, and at 12 months and 18 months post-MI. The vitality, mental health and mental component summaries showed significant improvements across time in the entire group (P < 0.02 to P < 0.04, two-factor ANOVA); no treatment or interaction effect was evident.
Figure 2.
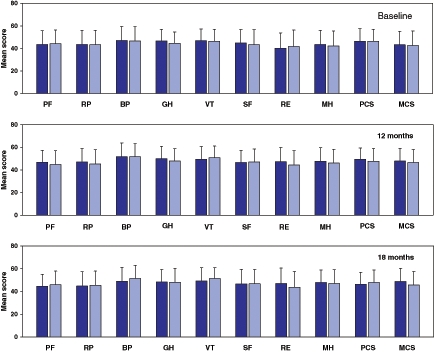
Norm-based scores for the eight domains of the Medical Outcomes Study 36-item Short Form health survey and the two summary scores in the intervention (dark bars) and the control (light bars) groups at three different time points during the study period. Error bars indicate SDs. BP Bodily pain; GH General health; MCS Mental component summary; MH Mental health; PCS Physical component summary; PF Physical functioning; RE Role emotional; RP Role physical; SF Social functioning; VT Vitality
DISCUSSION
A nurse-led case management approach has previously been shown to be effective (19–21) in the management of a variety of chronic medical conditions, but this approach has not extensively been used in North America. While there have been publications (22,23) of well-conducted and controlled trials examining the benefits of a nurse-managed approach in dyslipidemia, the present study unfortunately did not provide support for this type of approach in the follow-up and management of patients post-MI. This result of the present study was at variance with that of our previously published data (15) on an albeit small pilot project that was uncontrolled and conducted at a time when guidelines for lowering plasma lipid levels in secondary prevention were not as strongly supported as they are currently.
Despite the fact that the present study was powered to detect a clinically relevant difference in those patients achieving target LDL-C levels up to 18 months post-MI, that very few patients were lost in the course of the study and that the persistence in the use of lipid-lowering medication was surprisingly high, the study still demonstrated the existence of important treatment gaps. While our nurse-managed approach led to 87.3% of patients reaching plasma LDL-C levels of less than 2.5 mmol/L after repeated telephone interventions for patients and their primary care physicians over an average of 4.4 months post-MI, it should be noted that only 51.6% of patients were still at that goal 18 months after the index MI hospitalization. The percentage of patients reaching LDL-C levels of less than 2.5 mmol/L at 12 months and 18 months was not statistically different from that observed in the control group (95% CI for the difference for the two percentages included zero). This was despite the fact that no patients in the intervention group were left untreated, as opposed to 9% that were untreated in the control group (P < 0.03). These data suggest, however, that our nurse-managed approach, which incurred cost and time, was unsuccessful in improving lipid control beyond the extent currently achieved in primary care. Our data also suggest that there was probably conscious or unconscious reluctance to increase doses or initiate combination therapy to reach the target LDL-C level of less than 2.5 mmol/L. This reluctance may have been on both the primary physician side and on our own side, knowing that there is an increased risk of drug-induced rhabdomyolysis with higher doses of statins or in combination with fibrates (24). While higher doses of lipid-lowering drugs and more combination therapy were seen in the intervention group (Table 4), these small trends were not statistically significant and did not result in a higher proportion of patients reaching lipid targets.
Patients in both groups had obvious difficulty in achieving the lipid triple end point recommended by the 2000 Canadian consensus guidelines (16); only 23.4% and 38.3% of patients in the intervention and the control groups, respectively, were at that target 18 months after the index MI. Thus, despite motivated and compliant patients, well-trained nurse-managers, and conscientious primary care physicians and specialists, there were still obvious barriers in achieving and maintaining aggressive lipid targets after an MI. This appears to be true for the target LDL-C level, and doubly so for the goals of a TG level of less than 2.0 mmol/L and a TC:HDL-C ratio of less than 4.0. There could be several explanations for the difficulty in attaining the TG and TC:HDL-C ratio goals, including less scientific evidence demonstrating a benefit; possible excessive focus on LDL-C level lowering; drugs being less efficacious for increasing levels of HDL-C than for lowering those of LDL-C; the need for more complicated combination therapy; and intraindividual variability in plasma TG levels.
The apparent worsening of lipid values over the latter part of the follow-up period in both experimental groups is difficult to explain at the moment because doses of lipid-lowering agents appear to have remained relatively constant over time. This observation suggests that diet or lifestyle may have changed 12 to 18 months after a patient’s MI.
As for the intervention specifically tested in the present project, we were unable to demonstrate a benefit in terms of lipids, quality of life or longer-term adherence to lipid-lowering medications up to 18 months after the hospitalization for an MI. However, there are various possible explanations for the negative results. First, it is possible that within the means we had at our disposal, the intervention of nurse-centred surveillance and treatment was not sufficiently aggressive to impact our primary end point. This occurred despite the fact that the amount of time the nurse-manager spent conveying messages by telephone and trying to aggressively intervene on cardiovascular risk factors for patients in the intervention group was threefold longer than the time spent on patients in the control group. It is nevertheless surprising to realize that this intervention, minimal though it may be, did not result in any significant differences in the studied variables. Second, the clinical practice environment may have changed since we conducted our pilot experiment on this nurse-led management program (from 1997 to 1999) (15). It is likely that patients are currently being treated and followed more closely in primary care, or that our initial pilot experiment (15) heightened awareness of family physicians in our region and that such improvements eroded any impact of our nurse-managed approach. Third, it is possible that an eventual difference between the two groups may have been obscured by 23% of patients in both groups already taking lipid-lowering drugs on admission for their MI. Finally, it is possible that the study was not sufficiently powered to detect a smaller difference in the proportion of patients achieving lipid targets at 12 months and 18 months. This consideration notwithstanding, it is unlikely, given the results seen in the present trial, that our approach would have resulted in a favourable cost-benefit equation.
The data obtained in the present study may not entirely be generalized and may actually be system dependent, but they emphasize the importance of conducting controlled appraisals of this type of management approach before committing human and financial resources to larger and more expensive programs. The educational possibilities offered by programs like ours would seem to be important, but in an era of evidenced-based medicine, any such program should be fully evaluated before a commitment for possible substantial funding. The experience from the present study should encourage hospitals and other organizations to evaluate each component (comprehensiveness, duration, targeted population, etc) of large rehabilitation programs in an attempt to understand the exact impact of each component on end points, and to therefore ensure that a clear cost-benefit is achieved in programs aimed at modifying cardiovascular risk factors (or any similar clinical target). This is important in face of the growing trend for case management and rehabilitation programs in various therapeutic areas, both in Canada and elsewhere. This is especially important if these programs are targeting only a fraction of the high-risk population in which the per capita cost may be substantial.
ACKNOWLEDGMENTS
The authors acknowledge the support of Merck Frosst Canada and AstraZeneca (The Future Forum) for their unrestricted financial support. This study was also supported by a grant from the Heart and Stroke Foundation of Quebec and a Jonathan Ballon Research Award. The authors thank Mr Peter Hughes for his editorial assistance and Mr Gérald Lambert for his help in gathering some of the data for this paper.
REFERENCES
- 1.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 2.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 3.Grover SA, Coupal L, Paquet S, Zowall H. Cost-effectiveness of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in the secondary prevention of cardiovascular disease: Forecasting the incremental benefits of preventing coronary and cerebrovascular events. Arch Intern Med. 1999;159:593–600. doi: 10.1001/archinte.159.6.593. [DOI] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 5.Genest J, Frohlich J, Fodor G, McPherson R Working Group on Hypercholesterolemia and Other Dyslipidemias. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: Summary of the 2003 update. CMAJ. 2003;169:921–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Yarzebski J, Bujor CF, Goldberg RJ, Spencer F, Lessard D, Gore JM. A community-wide survey of physician practices and attitudes toward cholesterol management in patients with recent acute myocardial infarction. Arch Intern Med. 2002;162:797–804. doi: 10.1001/archinte.162.7.797. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Stafford RS, Ausiello JC, Chaisson CE. Randomized clinical trials and recent patterns in the use of statins. Am Heart J. 2001;141:957–63. doi: 10.1067/mhj.2001.115587. [DOI] [PubMed] [Google Scholar]
- 8.Velasco JA. After 4S, CARE and LIPID – Is evidence-based medicine being practised? Atherosclerosis. 1999;147(Suppl 1):S39–44. doi: 10.1016/s0021-9150(99)00254-3. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor GT, Buring JE, Yusuf S, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–44. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- 10.Cupples ME, McKnight A. Randomised controlled trial of health promotion in general practice for patients at high cardiovascular risk. BMJ. 1994;309:993–6. doi: 10.1136/bmj.309.6960.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120:721–9. doi: 10.7326/0003-4819-120-9-199405010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Alderman EL, Fair JM, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP) Circulation. 1994;89:975–90. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- 13.Bramlet DA, King H, Young L, Witt JR, Stoukides CA, Kaul AF. Management of hypercholesterolemia: Practice patterns for primary care providers and cardiologists. Am J Cardiol. 1997;80:39H–44H. doi: 10.1016/s0002-9149(97)00819-9. [DOI] [PubMed] [Google Scholar]
- 14.King KM, Humen DP, Teo KK. Cardiac rehabilitation: The forgotten intervention. Can J Cardiol. 1999;15:979–85. [PubMed] [Google Scholar]
- 15.Baillargeon JP, Lepage S, Larrivee L, Roy MA, Landry S, Maheux P. Intensive surveillance and treatment of dyslipidemia in the postinfarct patient: Evaluation of a nurse-oriented management approach. Can J Cardiol. 2001;17:169–75. [PubMed] [Google Scholar]
- 16.Fodor JG, Frohlich JJ, Genest JJ, Jr, McPherson PR. Recommendations for the management and treatment of dyslipidemia. Report of the Working Group on Hypercholesterolemia and Other Dyslipidemias. CMAJ. 2000;162:1441–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Hulley SB, Cummings SR.Designing Clinical Research: An Epidemiologic Approach 1Baltimore: Williams & Wilkins; 1988. 145 [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 19.Aubert RE, Herman WH, Waters J, et al. Nurse case management to improve glycemic control in diabetic patients in a health maintenance organization. A randomized, controlled trial. Ann Intern Med. 1998;129:605–12. doi: 10.7326/0003-4819-129-8-199810150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 21.Mundinger MO, Kane RL, Lenz ER, et al. Primary care outcomes in patients treated by nurse practitioners or physicians: A randomized trial. JAMA. 2000;283:59–68. doi: 10.1001/jama.283.1.59. [DOI] [PubMed] [Google Scholar]
- 22.Becker DM, Raqueno JV, Yook RM, et al. Nurse-mediated cholesterol management compared with enhanced primary care in siblings of individuals with premature coronary disease. Arch Intern Med. 1998;158:1533–9. doi: 10.1001/archinte.158.14.1533. [DOI] [PubMed] [Google Scholar]
- 23.Ammerman AS, Keyserling TC, Atwood JR, Hosking JD, Zayed H, Krasny C. A randomized controlled trial of a public health nurse directed treatment program for rural patients with high blood cholesterol. Prev Med. 2003;36:340–51. doi: 10.1016/s0091-7435(02)00042-7. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–90. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]