Abstract
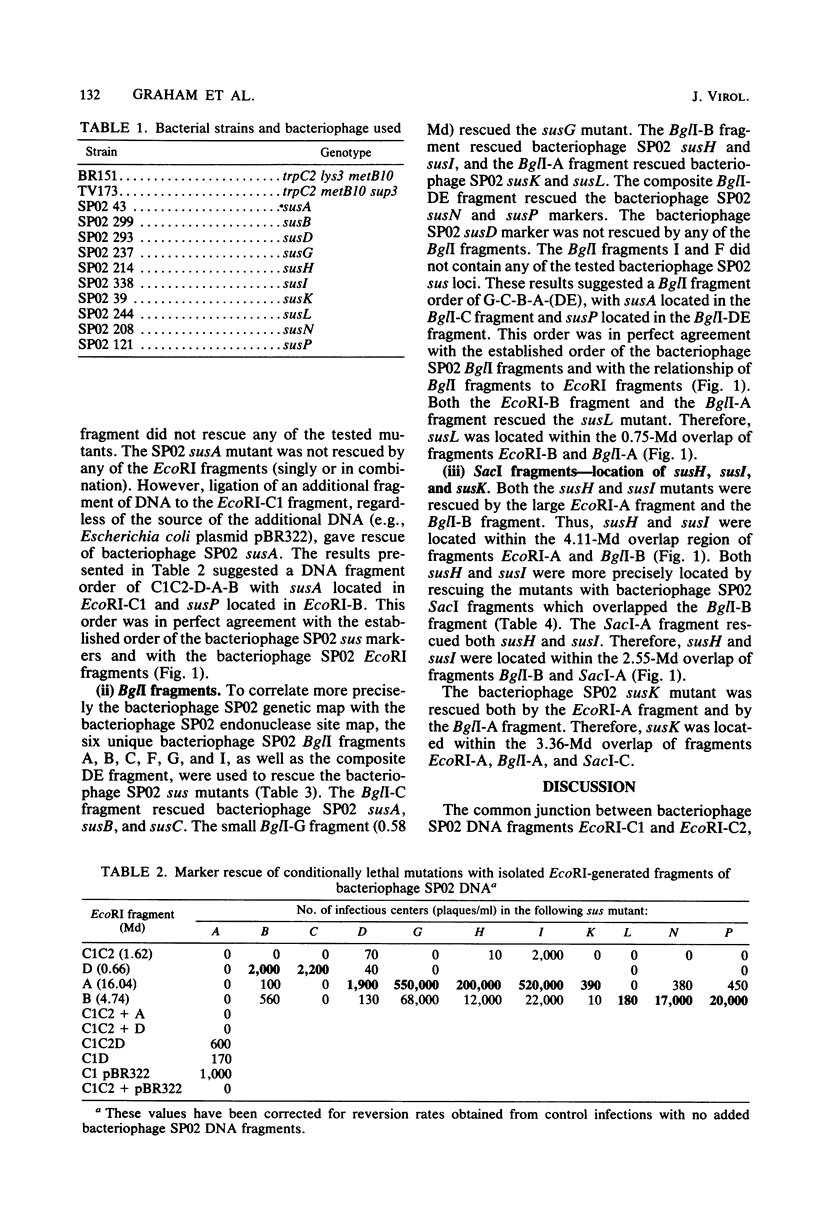
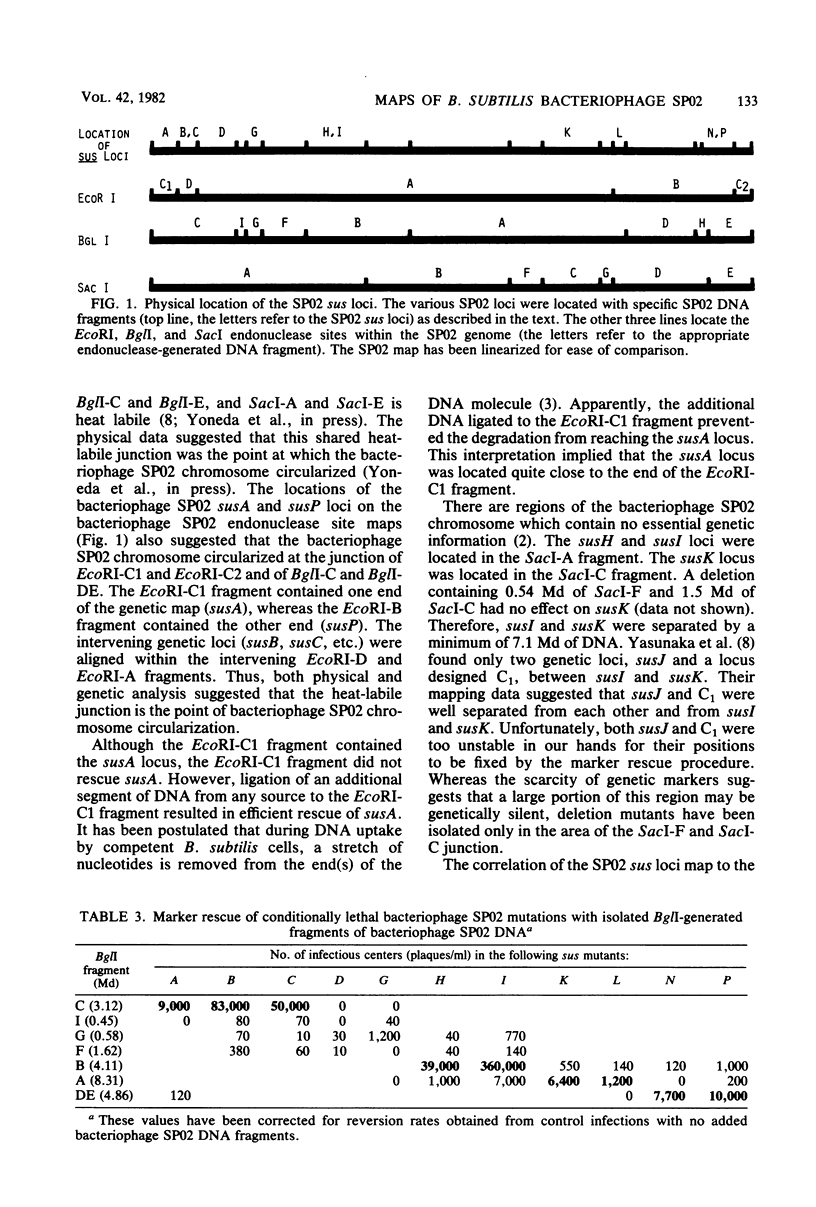
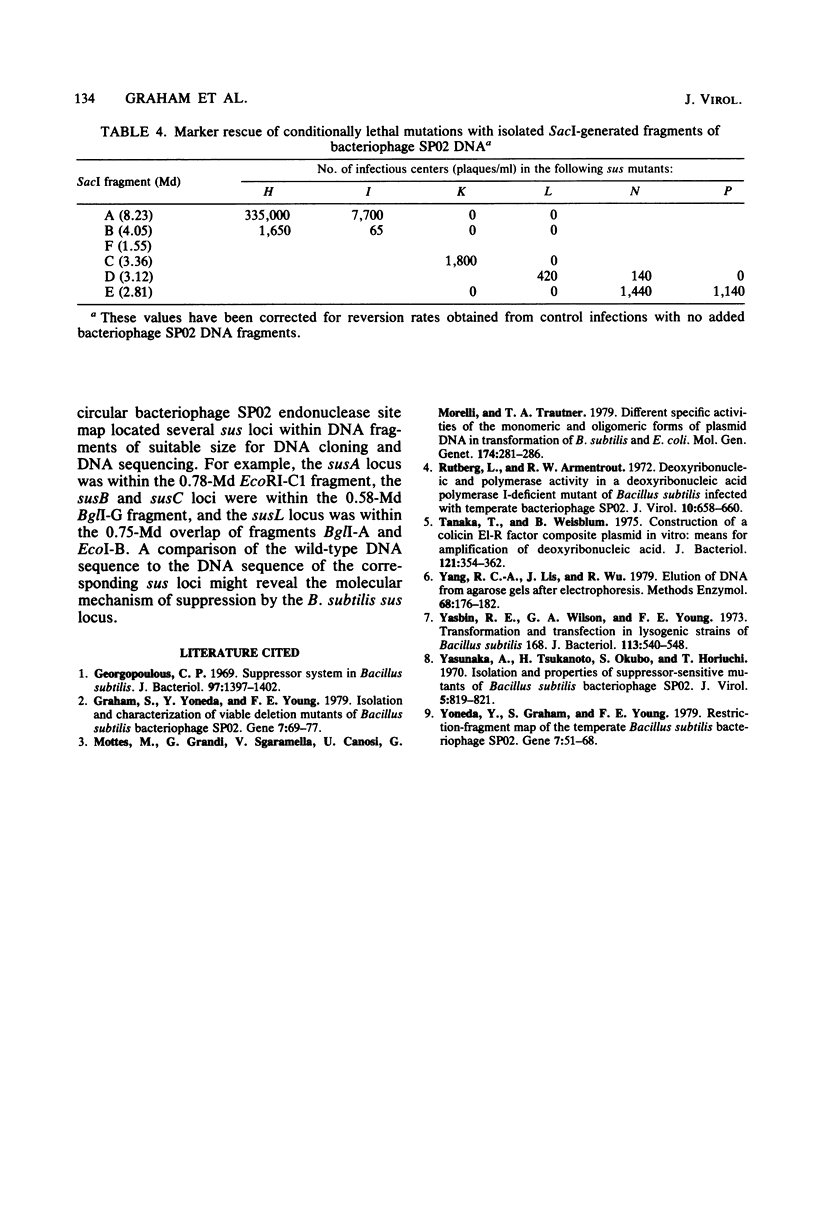
By marker rescue of bacteriophage SP02 sus mutants with purified bacteriophage SP02 DNA fragments, 11 of the 17 known bacteriophage SP02 sus loci were assigned to discrete DNA fragments. The left-most genetic locus, susA, was found to reside near one bacteriophage SP02 terminus (EcoRI-C1 fragment), whereas the right-most genetic locus, susP, was found to reside near the other bacteriophage SP02 terminus (EcoRI-C2 fragment). The physical locations of the intervening genetic loci were found to be consistent with the previously determined genetic order. Evidence was also obtained which suggested that at least one end of a transforming DNA fragment is degraded during DNA uptake by the competent bacterium.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Georgopoulos C. P. Suppressor system in Bacillus subtilis 168. J Bacteriol. 1969 Mar;97(3):1397–1402. doi: 10.1128/jb.97.3.1397-1402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S., Yoneda Y., Young F. E. Isolation and characterization of viable deletion mutants of Bacillus subtilis bacteriophage SPO2. Gene. 1979 Sep;7(1):69–77. doi: 10.1016/0378-1119(79)90043-x. [DOI] [PubMed] [Google Scholar]
- Mottes M., Grandi G., Sgaramella V., Canosi U., Morelli G., Trautner T. A. Different specific activities of the monomeric and oligomeric forms of plasmid DNA in transformation of B. subtilis and E. coli. Mol Gen Genet. 1979 Jul 24;174(3):281–286. doi: 10.1007/BF00267800. [DOI] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W. Deoxyribonucleic acid polymerase activity in a deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis infected with temperature bacteriophage SPO2. J Virol. 1972 Oct;10(4):658–660. doi: 10.1128/jvi.10.4.658-660.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaka K., Tsukamoto H., Okubo S., Horiuchi T. Isolation and properties of suppressor-sensitive mutants of Bacillus subtilis bacteriophage SP02. J Virol. 1970 Jun;5(6):819–821. doi: 10.1128/jvi.5.6.819-821.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Graham S., Young F. E. Restriction-fragment map of the temperate Bacillus subtilis bacteriophage SPO2. Gene. 1979 Sep;7(1):51–68. doi: 10.1016/0378-1119(79)90042-8. [DOI] [PubMed] [Google Scholar]


