Abstract
AIMS
While natriuretic peptides have demonstrated diagnostic and prognostic potential in cardiac disorders, little is known about their relationship with the onset and quantification of myocardial infarction. The relationship of serial N-terminal pro-brain natriuretic peptide (NT-proBNP) with duration from symptom onset, infarct size and prognosis in ST elevation myocardial infarction (STEMI) patients treated with primary percutaneous intervention was examined.
METHODS AND RESULTS
Three hundred thirty-one STEMI patients in the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial, which evaluated pexelizumab versus placebo, were studied. NT-proBNP (pg/mL) was measured at randomization, 24 h and 72 h; creatine kinase-MB area under the curve was measured at 72 h; and QRS score was assessed at discharge. Prognosis was ascertained from the 90-day composite clinical outcome of death, shock, stroke and congestive heart failure. Multivariate logistical regression was used to adjust for baseline characteristics for models at randomization, 24 h and 72 h. NT-proBNP was higher in patients with longer time from symptom onset (P<0.001) and correlated with measures of infarct size, including the area under the curve (P<0.001) and QRS score (P<0.001). Patients reaching the primary end point had markedly higher NT-proBNP at each sampling period (P<0.001). NT-proBNP at all time points was the strongest independent predictor of the primary end point in the multivariate model: in the 24 h model, only age and 24 h NT-proBNP (C-index 0.83); and only age, Killip class and NT-proBNP was in the 72 h model (C-index 0.85).
CONCLUSIONS
Higher NT-proBNP at 24 h correlated with larger infarct size and worse clinical outcomes. NT-proBNP at baseline, 24 h and 72 h after presentation with acute STEMI, is an independent predictor of a poor outcome and adds clinically useful prognostic information.
Keywords: Clinical trial, Myocardial infarction, Natriuretic peptides
Abstract
OBJECTIFS
Les peptides natriurétiques ont démontré un potentiel diagnostique et prospectif en présence de troubles cardiaques, mais on ne sait pas grand-chose de leur lien avec l’apparition et l’importance de l’infarctus du myocarde. Le lien du propeptide natriurétique cérébral N-terminal (NT-proBNP) avec la durée depuis l’apparition des symptômes, la dimension de l’infarctus et le pronostic chez les patients atteints d’un infarctus du myocarde avec surélévation du segment ST (IMSST) traités au moyen d’une intervention percutanée primaire a été examiné.
MÉTHODOLOGIE ET RÉSULTATS
Trois cent trente et un patients atteints d’un IMSST participant à l’étude COMMA sur l’inhibition complémentaire de l’infarctus du myocarde traités par angioplastie, qui évaluait le pexélizumab par rapport à un placebo, ont été étudiés. Le NT-proBNP (pg/mL) a été évalué à un moment aléatoire, au bout de 24 h, puis de 72 h, la zone de créatine-kinase MB sous la courbe a été mesurée à 72 h et l’indice de QRS a été évalué au congé. Le pronostic était déterminé d’après l’issue composite clinique de décès, de choc, d’accident vasculaire cérébral et d’insuffisance cardiaque congestive au bout de 90 jours. La régression logistique mutlivariée a permis de rajuster les caractéristiques de référence des modèles au moment aléatoire, à 24 h et à 72 h. Le NT-proBNP était plus élevé chez les patients ayant passé le plus de temps depuis l’apparition des symptômes (P<0,001) et était relié aux mesures de la dimension de l’infarctus, y compris la zone sous la courbe (P<0,001) et l’indice de QRS (P<0,001). Les patients atteignant le point de virage primaire présentaient un NT-proBNP considérablement plus élevé à chaque période d’échantillonnage (P<0,001). Le NT-proBNP à tous les points dans le temps était le prédicteur indépendant dominant du point de virage primaire dans le modèle multivarié; dans le modèle à 24 h, seuls l’âge et le NT-proBNP à 24 h (indice C 0,83) l’étaient, tandis qu’à 72 h, l’âge, la classe de Killip et le NT-proBNP (indice C 0,85) en faisaient partie.
CONCLUSIONS
Un NT-proBNP plus élevé à 24 h était lié à un plus gros infarctus et à des issues cliniques plus négatives. Le NT-proBNP au moment de référence, à 24 h et à 72 h après la présentation avec un IMSST aigu est un prédicteur indépendant d’issue négative et procure de l’information supplémentaire utile pour le pronostic.
The natriuretic peptide system is activated across a spectrum of cardiovascular disease that incorporates heart failure as well as the acute coronary syndromes, including ST segment elevation myocardial infarction (STEMI). Brain natriuretic peptide (BNP) is synthesized and released in a constitutive fashion by ventricular myocardium in response to increased wall tension and mechanical stretch, and is an ‘emergency’ cardiac hormone, involved in the regulation of sodium balance, blood pressure and blood volume (1). BNP is synthesized by cleavage of the 76 amino acid N-terminal (NT) portion of the prohormone (pro-BNP) to form the active molecule BNP and inactive molecule NT-proBNP (2). Elevated levels of both these molecules have been shown to confer powerful prognostic information in acute coronary syndromes (3,4), but there is limited information on the relationship of serial markers of the natriuretic system to STEMI. BNP (and NT-proBNP) rises quickly after the onset of infarction (5,6), and may be a marker of infarct-related artery (IRA) recanalization (7). However, the associations of tissue-level reperfusion, coronary artery flow and infarct size on BNP levels have not been adequately addressed. Furthermore, the relationship of baseline BNP and time of symptom onset in STEMI had not been studied. The issue of time from symptom onset has become increasingly important in the selection of therapeutic options (8); hence, BNP as a metric may play a key future role. We have accordingly evaluated the relationship between NT-proBNP levels and symptom onset, markers of reperfusion, size of infarct and prognosis in the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial (9).
PATIENTS AND METHODS
Study population
The COMMA trial, reported in detail previously, enrolled 960 patients with an acute myocardial infarction with either ST segment elevation or a new left bundle branch block. All 960 patients had symptoms less than 6 h, and primary angioplasty (percutaneous coronary intervention [PCI]) was planned as the primary mode of reperfusion therapy (9). Patients were randomly assigned to either placebo or one of two pexelizumab arms involving either a bolus or bolus and infusion during 24 h; patients in the current study were in either the placebo or the bolus and infusion pexelizumab arm due to availability of sample blood. This post hoc analysis represents 331 patients (175 from the placebo arm and 156 from the pexelizumab arm) in whom NT-proBNP was available. Blood had originally been sampled at randomization, 24 h and 72 h in the main trial for the determination of creatine kinase-MB (CK-MB) release and for pharmacodynamic and pharmacokinetic studies. The substudy patients are representative of the overall trial population in clinical, angiographic and outcome data (not shown).
End points
The primary end point used for this substudy analysis was the same as that used for the overall trial, ie, a 90-day composite end point of death, new or worsening heart failure, shock or disabling stroke. Information was available on all patients at 90 days.
Symptoms, infarct size and reperfusion
Symptoms were assessed by patient self-report and recorded on the case report forms by study staff. Symptoms are represented as the time from onset to the time of randomization, in minutes. Infarct size was assessed in three different ways: peak CK-MB (ng/mL), CK-MB area under the curve (AUC) (ng/mL/h) and QRS score at discharge (10). Myocardial reperfusion was assessed by ST segment resolution at 30 min and 24 h after PCI with categories of complete (greater than 70% resolution), partial (30% to 70% resolution) and none (less than 30% resolution) (11), and thrombolysis in myocardial infarction (TIMI) grade flow was assessed following PCI (0 to 3) by the attending physician (12). Core laboratories were used for analysis of electrocardiograms (EPICARE, USA) and blood samples (Montreal Heart Institute, Montreal, Quebec).
NT-proBNP
Samples were drawn at baseline (randomization), and at 24 h and 72 h postrandomization. Samples were allowed to clot for 35 min to 45 min, and the was blood centrifuged within 1 h of collection for 15 min at 3000 rpm, and shipped in dry ice to a central laboratory (Montreal Heart Institute, Montreal, Quebec) where it was stored at −70°C. Samples were batch analyzed at the end of the study by an electrochemiluminescence immunoassay with Roche Elecsys instrument and Elecsys proBNP assay kit (Roche Diagnostics, USA) with an analytical range of 5 pg/mL to 35,000 pg/mL, and intra-assay and interassay variability of 8% and 4%, respectively.
Statistical analysis
Baseline categorical variables are presented as percentages, and continuous variables are presented as medians with interquartile ranges (IQRs), unless otherwise stated. Categorical variables were compared using the χ2 test and continuous variables using the Mann-Whitney test. The Spearman correlation coefficient rho (ρ) was used to measure associations between discrete variables and NT-proBNP levels.
Multivariable, logistic regression using a backward, stepwise technique was used to adjust for baseline characteristics while assessing the relationship between NT-proBNP and prognosis. Baseline variables included in the model are those found in Table 1 and CK and CK-MB. Initially, a treatment variable was included but found to be nonsignificant, so it was excluded from subsequent analyses. NT-proBNP was divided into quartiles for logistic regression for the baseline model and treated as a continuous variable (per 1000 pg/mL increase) for the 24 h and 72 h models due to smaller sample size. Models were created for three different time points (0 h, 24 h and 72 h), and the C-statistic was used to examine the strength of the model. These models excluded patients (and their laboratory values) that had met the primary end point in the preceding time frame. All analyses were performed using SPSS 11.0 (SPSS Inc, USA) and P<0.05 was considered statistically significant.
TABLE 1.
Baseline characteristics of substudy population
| Characteristic | Overall population (n=331) | NT-proBNP below median (n=167) | NT-proBNP above median (n=174) | P |
|---|---|---|---|---|
| Age, years (range) | 61 (51–72) | 56 (49–65) | 68 (56–77) | <0.001 |
| Male sex | 76 | 82 | 69 | 0.005 |
| Prior MI | 16 | 13 | 19 | 0.29 |
| Prior heart failure | 10 | 5 | 16 | 0.002 |
| Diabetes | 20 | 18 | 23 | 0.27 |
| Prior CRVD | 7 | 2 | 12 | 0.001 |
| Smoking status | 65 | 69 | 61 | 0.03 |
| HR, beats/min (range) | 78 (66–89) | 76 (64–86) | 80 (68–92) | 0.006 |
| SBP, mmHg (range) | 131 (116–152) | 131 (118–148) | 130 (114–161) | 0.60 |
| DBP, mmHg (range) | 79 (68–92) | 80 (71–90) | 76 (65–94) | 0.12 |
| Killip class | 0.008 | |||
| I | 82 | 89 | 76 | |
| II | 15 | 11 | 20 | |
| III | 1 | 1 | 1 | |
| IV | 2 | 0 | 4 | |
| Anterior MI | 76 | 78 | 75 | 0.70 |
| Total ST elevation (range) | 1050 (600–1700) | 1050 (650–1688) | 1000 (600–1700) | 0.49 |
| ST segment resolution at 30 min, % | 0.16 | |||
| Complete | 42 | 50 | 34 | |
| Partial | 31 | 26 | 36 | |
| None | 18 | 14 | 22 | |
| ST segment resolution at 24 h, % | 0.07 | |||
| Complete | 53 | 60 | 46 | |
| Partial | 28 | 24 | 33 | |
| None | 9 | 7 | 11 | |
| QRS score (range) | ||||
| Baseline | 4 (2–7) | 3 (1–5) | 5 (2–7) | <0.001 |
| 24 h | 6 (3–10) | 6 (3–9) | 7 (4–10) | 0.06 |
| Discharge | 6 (3–9) | 6 (3–9) | 6 (4–9) | 0.32 |
| TIMI grade flow at 30 min | 0.79 | |||
| 0 or 1 | 5 | 4 | 6 | |
| 2 | 10 | 9 | 11 | |
| 3 | 85 | 87 | 83 |
Values are medians (interquartile range) or percentages. Smoking status includes current and former smokers. P refers to comparison of above and below median N-terminal pro-brain natriuretic peptide (NT-proBNP). BP Blood pressure; CRVD Cerebrovascular disease; D Diastolic; HR Heart rate; MI Myocardial infarction; S Systolic; TIMI Thrombolysis in myocardial infarction
RESULTS
The median age of patients was 61 years (IQR 51 to 72 years), 76% were men and 82% were Killip class I on presentation (Table 1). Patients with an NT-proBNP above the median were more likely to be older women, and have heart failure or cerebrovascular disease, a higher heart rate or worse Killip class on presentation (Table 1). ST segment resolution at 30 min was complete in 42%, partial in 31% and 18% had no resolution; at 24 h, ST segment resolution was complete in 53%, partial in 28% and 9% had no resolution. TIMI flow post-PCI for grade 0 or 1, 2 and 3 was 5%, 10% and 85%, respectively.
NT-proBNP and symptom duration
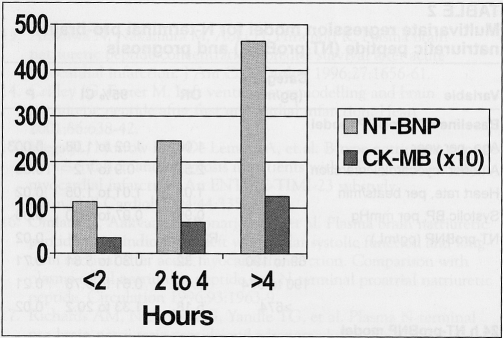
For patients with symptom duration of 2 h or shorter, 2 h to 4 h, or longer than 4 h, the respective baseline median (IQR) NT-proBNP concentration was 113 pg/mL (range 50 pg/mL to 245 pg/mL), 246 pg/mL (range 83 pg/mL to 711 pg/mL) and 464 pg/mL (range 158 pg/mL to 2174 pg/mL) (P<0.001 for trend) (Figure 1). Increasing NT-proBNP concentrations were positively correlated with increasing time from symptom onset to randomization, ρ=0.354 (P<0.001). Baseline CK-MB demonstrated a similar relationship (Figure 1) and correlation (ρ=0.328; P<0.001) with symptom duration. NT-proBNP was also correlated with CK-MB, ρ=0.402 (P<0.001) at baseline.
Figure 1.
N-terminal pro-brain natriuretic peptide (NT-BNP) (light grey bars, pg/mL) and creatine kinase-MB (CK-MB) (dark grey bars, ng/mL ×10) concentrations in relation to symptom onset, in hours
NT-proBNP, infarct size and evidence of reperfusion
NT-proBNP at 24 h was correlated with peak CK-MB (ρ=0.40; P<0.001), CK-MB AUC (ρ=0.45; P<0.001) and QRS score at discharge (ρ=0.40; P<0.001). Greater percentage ST segment resolution at 24 h was associated with a lower 24 h NT-proBNP (ρ= −0.14; P=0.02), whereas 24 h NT-proBNP was not correlated with initial ST segment resolution at 30 min (P=0.54), but was inversely correlated with TIMI grade flow post-PCI (ρ= −0.13; P=0.02) (ie, higher grade TIMI flow was associated with a lower NT-proBNP).
NT-proBNP and mortality
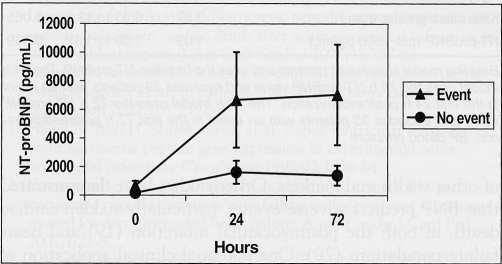
The composite end point was met by 46 (13.9%) of 331 patients in the substudy, which included 20 (6.0%) deaths, 21 (6.3%) with shock, one (0.3%) stroke and 22 (6.6%) with new-onset heart failure. Of 21 patients who developed shock, 11 died, as did three of the patients who developed heart failure. Patients who had an event had a significantly higher median NT-proBNP at all three time points compared with those who remained event-free (P<0.05; Figure 2).
Figure 2.
N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations at 0 h, 24 h and 72 h for those who had or did not have an event up to 90 days. Error bars represent 25th and 75th percentiles
In a logistical regression model that included NT-proBNP (and adjusting for important clinical variables), higher baseline NT-proBNP predicted a poor outcome (C-index 0.82), as did the 24 h and 72 h NT-proBNP (C-index 0.83 and 0.85, respectively) (Table 2). In the model at 24 h, only NT-proBNP and age were significant independent predictors, while at 72 h, only NT-proBNP, initial Killip class and age were predictors of a poor outcome at 90 days.
TABLE 2.
Multivariate regression model for N-terminal pro-brain natriuretic peptide (NT-proBNP) and prognosis
| Variable | Category (pg/mL) | OR | 95% CI | P |
|---|---|---|---|---|
| Baseline NT-proBNP model | ||||
| Age, per year | 1.06 | 1.02 to 1.08 | 0.003 | |
| Anterior myocardial infarction | 2.55 | 0.9 to 7.2 | 0.08 | |
| Heart rate, per beats/min | 1.03 | 1.01 to 1.05 | 0.02 | |
| Systolic BP, per mmHg | 0.99 | 0.97 to 1.00 | 0.03 | |
| NT-proBNP (pg/mL) | <69 | Referent | 0.02 | |
| 69 to 190 | 1.32 | 0.30 to 5.81 | 0.71 | |
| 190 to 674 | 2.45 | 0.61 to 9.78 | 0.21 | |
| >674 | 5.18 | 1.33 to 20.2 | 0.02 | |
| 24 h NT-proBNP model | ||||
| Age, per year | 1.06 | 1.01 to 1.12 | 0.013 | |
| NT-proBNP (per 1000 pg/mL) | 1.09 | 1.02 to 1.17 | 0.014 | |
| 72 h NT-proBNP model | ||||
| Age, per year | 1.05 | 0.99 to 1.12 | 0.078 | |
| Killip class greater than I | 3.52 | 0.93 to 13.3 | 0.065 | |
| NT-proBNP (per 1000 pg/mL) | 1.09 | 1.00 to 1.19 | 0.039 | |
Baseline model includes all patients and uses the baseline NT-proBNP. The 24 h model uses the 24 h NT-proBNP value and excludes 29 patients with an event in the first 24 h postrandomization. The 72 h model uses the 72 h NT-proBNP value and excludes 35 patients with an event in the first 72 h postrandomization. BP Blood pressure
DISCUSSION
Our observations reveal NT-proBNP to be an independent and potent prognostic risk factor – more powerful than traditional markers of risk – at three early time points in patients presenting with STEMI and treated with primary PCI. We have demonstrated three novel findings: the basal level of NT-proBNP is proportionate to the elapsed time from symptom onset in patients presenting with STEMI; NT-proBNP is correlated with infarct size as determined by biochemical and electrocardiographic measures; and NT-proBNP is closely related to measures of successful reperfusion.
Prognosis
Other studies have demonstrated a similar pattern of risk for patients with elevated NT-proBNP (5,6,13–18). Our study extends these prior findings by demonstrating that, at baseline, 24 h and 72 h, NT-proBNP predicts adverse events independent of other traditional markers. Other studies have demonstrated that BNP predicts adverse events, particularly sudden cardiac death, in both the postmyocardial infarction (19) and heart failure populations (20). One potential clinical application of natriuretic peptides, yet to be tested, is to stratify patients according to their sudden cardiac death risk and to identify, together with other clinical variables, who may benefit from implantation of a cardioverter defibrillator. Finally, NT-proBNP was predictive of a higher risk for subsequent events in the very early phases of an acute infarct – this may help direct further research into the delivery of more invasive therapy, such as direct percutaneous intervention or transfer for PCI, by selecting those who would derive the most benefit from these therapies.
Size of infarct
No prior study has looked at the size of infarct as determined by traditional markers of peak CK-MB or CK-MB AUC, or novel markers such as QRS score at discharge. In the only other contemporary study of natriuretic peptides in STEMI (15), baseline BNP was not found to be correlated with CK-MB, which was possibly due to the fact that only one-third of patients had anterior myocardial infarctions. We demonstrated that the size of infarct was in fact proportional to the level of NT-proBNP in a high-risk population with a larger extent of muscle at risk for necrosis. Mechanistically, this may relate to the release of presynthesized BNP from necrosing areas, an increase in messenger RNA in peri-infarct myocytes or increased wall stress in the remaining viable peri-infarct myocardium (21). Although no conclusive evidence exists, elevated NT-proBNP may indicate that there is more viable myocardium surrounding the infarct zone, and this elevation in NT-proBNP may portend an increased risk of adverse outcome because the peri-infarct zones are more likely to produce a lethal arrhythmia than non-infarcted tissue or scar tissue.
Duration of symptoms and NT-proBNP
We demonstrated a clear relationship between the elapsed time from symptom onset and the plasma concentration of NT-proBNP ascertained at presentation to medical care. As in the case in heart failure, NT-proBNP in the setting of STEMI likely represents, at least in part, the left ventricular filling pressure (22). The timing of symptom onset of a STEMI, although not always concordant with epicardial coronary occlusion, plays an integral role in the decision process of which reperfusion strategy to choose. Hence, natriuretic peptides measurement could play a useful role in this decision process, especially if the timing of symptom onset is in question, or if further immediate risk-stratification information is sought.
Tissue perfusion and IRA flow
We demonstrated, for the first time, that the tissue-level perfusion as measured by ST segment resolution at 24 h was associated with lower NT-proBNP levels. Prior studies of STEMI and BNP have not examined tissue-level perfusion. We also demonstrated that increased TIMI grade flow at 30 min was related to lower subsequent NT-proBNP levels. One prior study (7) of 80 patients evaluated the relationship between the IRA status and BNP following facilitated PCI and found no relationship between patency of the IRA and BNP levels. However, the study had substantial variation among time of symptom onset, treatment received and patients excluded before analysis (7).
Limitations
Some limitations to our study should be noted. First, although we have demonstrated that NT-proBNP within the first 72 h has powerful prognostic information after an acute myocardial infarct, it is unclear whether the same relationship will be evident if NT-proBNP is assessed later. However, elevated natriuretic peptides after STEMI have consistently demonstrated a strong, negative prognostic value even up to two years (17). Second, we chose to treat NT-proBNP as a continuous variable and in quartiles rather than use a specified cutpoint as is done elsewhere (15). However, the prespecified cutpoint of 80 pg/mL for BNP (not NT-proBNP) was originally developed for aiding the diagnosis of heart failure and may not be relevant for the prognosis of an acute myocardial infarction population; it is likely that a clinically useful cutpoint will vary based on myocardial territory involved (anterior versus inferior) (6), and other factors including age and sex (23), renal function (24) and body mass (25).
CONCLUSIONS
We have demonstrated that higher NT-proBNP is correlated with longer time from symptom onset to presentation, worse tissue-level perfusion and larger infarct size in patients with acute STEMI treated with primary PCI. NT-proBNP has a potential role in early risk stratification of patients during STEMI, mainly for sizing the infarct but possibly for determining the status of reperfusion. Finally, early and sequential assessment of NT-proBNP may be helpful in determining optimal therapeutic strategies given the key role of time in patient decisions.
ACKNOWLEDGEMENTS
The present study was supported by Procter & Gamble Pharmaceuticals (USA) and Alexion Pharmaceuticals, Inc (USA).
REFERENCES
- 1.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 2.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–22. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 3.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–21. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 4.James SK, Lindahl B, Siegbahn A, et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: A Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation. 2003;108:275–81. doi: 10.1161/01.CIR.0000079170.10579.DC. [DOI] [PubMed] [Google Scholar]
- 5.Morita E, Yasue H, Yoshimura M, et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation. 1993;88:82–91. doi: 10.1161/01.cir.88.1.82. [DOI] [PubMed] [Google Scholar]
- 6.Talwar S, Squire IB, Downie PF, et al. Profile of plasma N-terminal proBNP following acute myocardial infarction; correlation with left ventricular systolic dysfunction. Eur Heart J. 2000;21:1514–21. doi: 10.1053/euhj.1999.2045. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Sakuma M, Yaguchi I, et al. Early recanalization and plasma brain natriuretic peptide as an indicator of left ventricular function after acute myocardial infarction. Am Heart J. 2002;143:790–6. doi: 10.1067/mhj.2002.122170. [DOI] [PubMed] [Google Scholar]
- 8.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 9.Granger CB, Mahaffey KW, Weaver WD, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: The COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–90. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 10.Hindman NB, Schocken DD, Widmann M, et al. Evaluation of a QRS scoring system for estimating infarct size. V. Specificity and method of application of the complete system. Am J Cardiol. 1985;55:1485–90. doi: 10.1016/0002-9149(85)90958-0. [DOI] [PubMed] [Google Scholar]
- 11.Schroder R, Wegscheider K, Schroder K, Dissmann R, Meyer-Sabellek W. Extent of early ST segment elevation resolution: A strong predictor of outcome in patients with acute myocardial infarction and a sensitive measure to compare thrombolytic regimens. A substudy of the International Joint Efficacy Comparison of Thrombolytics (INJECT) trial. J Am Coll Cardiol. 1995;26:1657–64. doi: 10.1016/0735-1097(95)00372-x. [DOI] [PubMed] [Google Scholar]
- 12.Passamani E. The Thrombolysis In Myocardial Infarction (TIMI) trial. N Engl J Med. 1985;312:932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa N, Nakamura M, Aoki H, Hiramori K. Plasma brain natriuretic peptide concentrations predict survival after acute myocardial infarction. J Am Coll Cardiol. 1996;27:1656–61. doi: 10.1016/0735-1097(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 14.Crilley JG, Farrer M. Left ventricular remodelling and brain natriuretic peptide after first myocardial infarction. Heart. 2001;86:638–42. doi: 10.1136/heart.86.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mega JL, Morrow DA, De Lemos JA, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: An ENTIRE-TIMI-23 substudy. J Am Coll Cardiol. 2004;44:335–9. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Omland T, Aakvaag A, Bonarjee VV, et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93:1963–9. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 17.Richards AM, Nicholls MG, Yandle TG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: New neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998;97:1921–9. doi: 10.1161/01.cir.97.19.1921. [DOI] [PubMed] [Google Scholar]
- 18.Richards AM, Nicholls MG, Espiner EA, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–92. doi: 10.1161/01.CIR.0000070953.76250.B9. [DOI] [PubMed] [Google Scholar]
- 19.Tapanainen JM, Lindgren KS, Makikallio TH, Vuolteenaho O, Leppaluoto J, Huikuri HV. Natriuretic peptides as predictors of non-sudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2004;43:757–63. doi: 10.1016/j.jacc.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Berger R, Huelsman M, Strecker K, et al. B-Type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–7. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 21.Hama N, Itoh H, Shirakami G, et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation. 1995;92:1558–64. doi: 10.1161/01.cir.92.6.1558. [DOI] [PubMed] [Google Scholar]
- 22.Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: A pilot study. J Card Fail. 2001;7:21–9. doi: 10.1054/jcaf.2001.23355. [DOI] [PubMed] [Google Scholar]
- 23.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: Impact of age and gender. J Am Coll Cardiol. 2002;40:976–82. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 24.McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: An analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41:571–9. doi: 10.1053/ajkd.2003.50118. [DOI] [PubMed] [Google Scholar]
- 25.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]