Abstract
BACKGROUND AND OBJECTIVES
To examine the capacity of angina and related symptoms experienced during exercise-stress testing to detect the presence of ischemia, controlling for other clinical factors.
METHOD
The authors undertook a prospective study of 482 women and 425 men (mean age 58 years) undergoing exercise stress testing with myocardial perfusion imaging. One hundred forty-six women and 127 men reported chest pain, and of these, 25% of women and 66% of men had myocardial perfusion imaging evidence of ischemia during testing. The present article focuses on patients with chest pain during testing.
MAIN OUTCOME MEASURES
Outcome measures included chest pain localization, extension, intensity and quality, as well as the presence of various nonpain-related symptoms. Backward logistical regression analyses were performed separately on men and women who had experienced chest pain during testing.
RESULTS
Men who described their chest pain as ‘heavy’ were 4.6 times more likely to experience ischemia during testing (P=0.039) compared with other men, but this pain descriptor only slightly improved accuracy of prediction beyond that provided by control variables. In women, several symptoms added to the sensitivity of the prediction, such as a numb feeling in the face or neck region (OR 4.5; P=0.048), a numb feeling in the chest area (OR 14.6; P=0.003), muscle tension (OR 5.2; P=0.013), and chest pain that was described as hot or burning (OR 4.3; P=0.014).
CONCLUSIONS
A more refined evaluation of symptoms experienced during testing was particularly helpful in improving detection of ischemia in women, but not in men. Attention to these symptoms may favour timely diagnosis of myocardial perfusion defects in women.
Keywords: Angina, Coronary disease, Imaging, Ischemia, Sex
Abstract
HISTORIQUE ET OBJECTIFS
Examiner la capacité de l’angine et des symptômes connexes ressentis pendant une épreuve à l’effort à déceler la présence d’ischémie, afin de contrôler d’autres facteurs cliniques.
MÉTHODOLOGIE
Les auteurs ont entrepris une étude prospective auprès de 482 femmes et de 425 hommes (âge moyen de 58 ans) subissant une épreuve à l’effort accompagnée d’une imagerie de perfusion myocardique. Cent quarante-six femmes et 127 hommes ont déclaré ressentir des douleurs thoraciques. De ce nombre, 25 % des femmes et 66 % des hommes présentaient des signes d’ischémie à l’imagerie de perfusion myocardique pendant l’épreuve. Le manuscrit porte sur les patients ressentant des douleurs thoraciques pendant l’épreuve à l’effort.
PRINCIPALES MESURES D’ISSUES
Les mesures d’issues incluent le foyer, l’étendue, l’intensité et la qualité des douleurs thoraciques, de même que la présence de divers symptômes non reliés à la douleur. Des analyses de régression logistique inversée ont été exécutées séparément chez les hommes et les femmes qui avaient ressenti des douleurs thoraciques pendant l’épreuve à l’effort.
RÉSULTATS
Les hommes qui désignaient leurs douleurs thoraciques d’ « importantes » risquaient 4,6 fois plus de souffrir d’ischémie pendant l’épreuve (P=0,039) que les autres hommes, mais ce descripteur de la douleur n’assurait qu’une légère augmentation de la précision par rapport à celle assurée par les variables de contrôle. Chez les femmes, plusieurs symptômes contribuaient à la sensibilité prévisionnelle, tels qu’un engourdissement de la région du visage et du cou (RR 4,5, P=0,048), un engourdissement de la région thoracique (RR 14,6; P=0,003), une tension musculaire (RR 5,2; P=0,013) et des douleurs thoraciques qualifiées de brûlantes ou de cuisantes (RR 4,3; P=0,014).
CONCLUSIONS
Une évaluation plus perfectionnée des symptômes ressentis pendant l’épreuve à l’effort était particulièrement utile pour améliorer le dépistage de l’ischémie chez les femmes, mais pas chez les hommes. Une attention accordée à ces symptômes pourrait favoriser un diagnostic rapide d’anomalies de la perfusion myocardique chez les femmes.
Coronary artery disease (CAD) is the leading cause of mortality in men and women in industrialized societies (1). However, there is growing evidence for some sex differences in the type or frequency of symptoms associated with CAD (2,3). For example, we have found that women with evidence of exercise-induced myocardial perfusion defects report more intense pain, use different words to describe their pain and experience more nonpain symptoms compared with men (4). The interpretation of these differences may constitute a barrier to the proper diagnosis of CAD in women given that the standard diagnostic criteria of acute coronary events are still primarily those developed from research with men.
The studies (3) that have examined sex differences in CAD symptomatology have typically contained various limitations. Most prior research has used a retrospective design, examined a limited number of nonpain symptoms, and has not controlled for pertinent clinical risk factors. Our recent study (4) addressed these elements but a concern remains that investigations have performed analyses only on patients with documented acute coronary syndromes (ACS) (usually, unstable angina and/or myocardial infarction [MI]). Sex differences in symptoms among these samples have been presumed to suggest sex differences in symptoms of CAD. However, while these women may experience more or different symptomatology, it is possible that not all symptoms are CAD-related. Indeed, in a previous study (2) on patients presenting to the emergency department (ED) with nontraumatic chest pain, it was shown, for example, that women more often reported pain in the back. However, this symptom was not predictive of the presence of ischemia in women on follow-up diagnostic testing.
We conducted a prospective, standardized investigation of a broad range of symptoms experienced by men and women with suspected or stable CAD undergoing exercise stress testing with myocardial perfusion imaging (MPI). Sex differences in the experience of pain and other symptoms in the patients with angina and MPI evidence of ischemia during testing have already been reported (4). In the current paper, analyses were repeated with a larger sample that included both ischemic and nonischemic patients. The goal of these analyses was to determine which symptoms are useful in detecting presence of ischemia on testing in men and women, after controlling for pertinent clinical variables. Based on previous findings (2) in patients presenting to the ED with nontraumatic chest pain, we anticipated that atypical/nonpain symptoms will be particularly helpful in improving detection of ischemia in women, but not in men.
METHODS
The research protocol was accepted by the Hospital Scientific and Ethics Committees, and focused on sex differences in symptomatology during exercise stress testing. The study took place between 2000 and 2003.
Patients
Nine hundred seven consecutive and consenting patients (425 men and 482 women) referred to a tertiary hospital specializing in cardiology for exercise stress assessment using MPI with technetium-99m-sestamibi single photon emission tomography (SPECT) were recruited on the first day of their examination. They were referred for testing principally for screening purposes and evaluation of stable CAD.
Patients were excluded from participation if they did not understand, speak and read French or English, were younger than 18 years, were in-patients, had a documented cardiac event (MI) or procedure (eg, angioplasty) in the previous two months, had another significant and incapacitating health problem (eg, cancer or chronic pain syndrome), reported a substance abuse problem or had an apparent cognitive deficit.
Fifty-five men and 58 women refused to participate for the following reasons: lack of time (n=12), not interested (n=38), too tired, too stressed or uncomfortable to participate (n=10), or no precise reason (n=53). Age could only be obtained from 52 of the 113 participants who declined to participate (mean age 60 years). The board of ethics refused authorization to obtain additional medical information from these patients’ charts.
Demographic and medical characteristics of the participating patients with chest pain are listed in Tables 1 and 2.
TABLE 1.
Sociodemographic profile of men and women with pain during exercise single photon emission tomography
| Men without ischemia (n=48)
|
Men with ischemia (n=94)
|
Women without ischemia (n=119)
|
Women with ischemia (n=38)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | P | n | % | n | % | P |
| Marital status | 0.445 | 0.154 | ||||||||
| Single | 4 | 8 | 6 | 6 | 12 | 10 | 3 | 8 | ||
| Married/living with someone | 35 | 73 | 77 | 82 | 71 | 60 | 17 | 45 | ||
| Separated/divorced/widowed | 9 | 19 | 11 | 12 | 36 | 30 | 18 | 47 | ||
| Currently employed | 31 | 65 | 39 | 42 | 0.009 | 57 | 48 | 15 | 40 | 0.364 |
| Education | 0.695 | 0.427 | ||||||||
| Elementary | 9 | 19 | 17 | 18 | 31 | 26 | 14 | 37 | ||
| High school | 22 | 46 | 36 | 38 | 45 | 38 | 13 | 34 | ||
| College/university | 17 | 35 | 39 | 42 | 43 | 36 | 11 | 29 | ||
TABLE 2.
Medical profile of men and women with pain during exercise single photon emission tomography
| Variable | Men without ischemia (n=48) | Men with ischemia (n=94) | P | Women without ischemia (n=119) | Women with ischemia (n=38) | P |
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 57.29±9.62 | 61.24±9.02 | 0.017 | 56.47±9.34 | 62.00±9.06 | 0.002 |
| Body mass index, kg/m2 (mean ± SD) | 27.46±3.16 | 28.00±3.90 | 0.407 | 26.15±4.13 | 26.51±3.68 | 0.635 |
| Smoker, n (%) | 6 (12.5) | 15 (16.0) | 0.583 | 14 (11.8) | 4 (10.5) | 0.835 |
| History of angina, n (%) | 21 (43.8) | 59 (62.8) | 0.097 | 42 (35.3) | 19 (50.0) | 0.244 |
| History of coronary events/procedures, n (%) | 22 (45.8) | 57 (60.6) | 0.093 | 19 (16.0) | 11 (28.9) | 0.076 |
| Hypertension, n (%) | 24 (50.0) | 47 (50.0) | 1.000 | 58 (48.7) | 15 (39.5) | 0.495 |
| Diabetes, n (%) | 5 (10.4) | 20 (21.3) | 0.203 | 9 (7.6) | 2 (5.3) | 0.629 |
| Hypercholesterolemia, n (%) | 29 (60.4) | 58 (61.7) | 0.569 | 48 (40.3) | 22 (57.9) | 0.113 |
| Hypothyroidism, n (%) | 1 (2.1) | 4 (4.3) | 0.507 | 24 (20.2) | 7 (18.4) | 0.689 |
| Family history of heart disease, n (%) | 36 (75.0) | 72 (76.6) | 0.748 | 101 (84.9) | 30 (78.9) | 0.223 |
| Family history of hypertension, n (%) | 23 (47.9) | 37 (39.4) | 0.355 | 71 (59.7) | 21 (55.3) | 0.480 |
| Family history of diabetes, n (%) | 13 (27.1) | 31 (33.0) | 0.448 | 47 (39.5) | 22 (57.9) | 0.062 |
| Beta-blockers, n (%) | 15 (31.3) | 54 (57.4) | 0.003 | 37 (31.1) | 18 (47.4) | 0.041 |
| Calcium blockers, n (%) | 10 (20.8) | 32 (34.0) | 0.101 | 25 (21.0) | 10 (26.3) | 0.423 |
| Anticoagulants, n (%) | 26 (54.2) | 70 (74.5) | 0.012 | 48 (40.3) | 20 (52.6) | 0.116 |
| Lipid-lowering agents, n (%) | 25 (52.1) | 60 (63.8) | 0.169 | 31 (26.1) | 13 (34.2) | 0.265 |
| Patients tested in the morning, n (%) | 26 (54.2) | 66 (70.2) | 0.058 | 76 (63.9) | 22 (57.9) | 0.470 |
| METS during exercise testing (mean ± SD) | 8.31±1.73 | 7.4±1.63 | 0.002 | 6.66±1.42 | 5.79±1.65 | 0.002 |
| Duration of exercise testing, s (mean ± SD) | 460±103 | 399±99 | 0.001 | 352±88 | 297±103 | 0.002 |
| Peak heart rate during exercise (mean ± SD) | 137±24 | 127±24 | 0.015 | 143±23 | 133±25 | 0.017 |
| Peak systolic BP during exercise, mmHg (mean ± SD) | 177±26 | 171±26 | 0.218 | 163±23 | 162±37 | 0.864 |
| Peak diastolic BP during exercise, mmHg (mean ± SD) | 87±12 | 88±12 | 0.642 | 86±11 | 83±17 | 0.176 |
| Canadian angina classification, n (%) | 0.275 | 0.741 | ||||
| Class I | 1 (2.1) | 3 (3.2) | 8 (6.7) | 1 (2.6) | ||
| Class II | 25 (52.1) | 39 (41.5) | 56 (47.1) | 17 (44.7) | ||
| Class III | 3 (6.3) | 9 (9.6) | 8 (6.7) | 5 (13.2) | ||
| Class IV | 0 (0.0) | 5 (5.3) | 6 (5.0) | 3 (7.9) | ||
| Type of daily angina pain or discomfort, n (%) | 0.092 | 0.057 | ||||
| Pain free | 13 (27.1) | 39 (41.5) | 30 (25.2) | 14 (36.8) | ||
| Typical pain | 11 (22.9) | 22 (23.4) | 24 (20.2) | 13 (34.2) | ||
| Atypical pain | 14 (29.2) | 26 (27.7) | 38 (31.9) | 7 (18.4) | ||
| Nonspecific pain | 9 (18.8) | 6 (6.4) | 26 (21.8) | 4 (10.5) |
BP Blood pressure; METS Metabolic estimated cost
Procedure
Patients scheduled to undergo exercise testing with MPI were required to make two appointments: a baseline session and an exercise session. Consenting patients participated in a brief interview and completed various questionnaires on the day of their baseline scan to obtain information on their medical, sociodemographic and psychological status. On the following day, immediately postexercise and before their scan, participants were questioned as to the location of any pain or discomfort, the qualitative aspects and intensity of this pain or discomfort, and any other symptoms experienced during exercise testing. Note that at the time of the interviews, the research assistants were blind as to whether the participants had experienced ischemia during the exercise protocol.
Instruments
Dermatome Pain Map
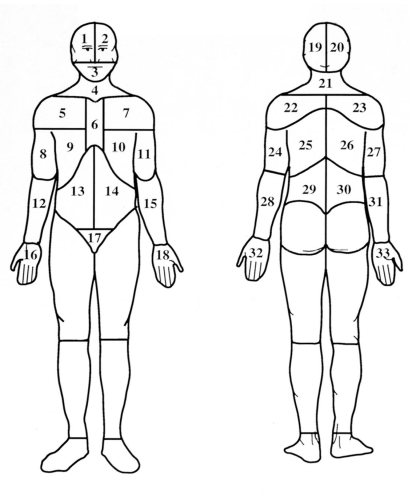
The Dermatome Pain Map (DPM) (5,6) includes two line drawings of the human body and is designed to assess anatomical pain location. Patients were asked to shade in the spatial distribution of their pain or discomfort. For the purpose of scoring, the figures were then divided into 33 anterior (front) and posterior (back) areas using an acetate (Figure 1). A point was given for a specific area if that area overlapped with the patient’s drawing. Extension of the pain was quantified by counting the number of areas involved.
Figure 1.
Dermatome Pain Map
Short form of the McGill Pain Questionnaire
The McGill Pain Questionnaire (MPQ) (7) measures the qualitative and quantitative aspects of pain and consists of 15 descriptors (11 sensory, four affective), rated on a scale of 0 (no pain) to 3 (severe pain). Participants were asked to choose the words that described their pain or discomfort, and to rate the intensity of that particular quality. The sum of the intensity ratings produced three scores: sensory, affective and total scores (sum of all descriptors). For the purposes of the present study, four other sensory descriptors related to angina pain (suffocating, tight, pressing and radiating), taken from the long form of the MPQ (8), were added to the short form MPQ as many patients in a prior investigation (2) had spontaneously reported them. The present pain index of the short form MPQ provided another measure of pain intensity.
Symptom checklist
The symptom checklist is a 27-item house instrument designed to assess the more subtle symptoms associated with ischemia. It includes symptoms typically associated with angina (eg, breathlessness and tight feeling in the chest) and symptoms said to be more prevalent in women (eg, nausea and fatigue). Some symptoms (eg, runny eyes) not expected to be associated with ischemia were added to ensure patients were not simply over-reporting symptoms. Lastly, the participant could add other symptoms if not found on the list. The chosen symptoms were derived from a review of the literature, as well as a perusal of various French and English symptom checklists to identify terms that had identical meaning in both languages. A backward translation was performed by three judges to ensure that the chosen terms were appropriate. This approach ensured greater transcultural validity of this measure.
Chest pain quality scale
The chest pain quality scale was employed by research assistants to classify patients’ daily chest pain as typical of angina, atypical or nonspecific. Typical angina is defined as chest pain that meets the following three criteria: precipitated by effort, located retrosternally, and is promptly relieved by rest or nitroglycerine. Atypical angina meets two of three criteria, while nonspecific chest pain meets one or none of the criteria.
Canadian Cardiovascular Society grading scale of angina pectoris
The Canadian Cardiovascular Society grading scale of angina pectoris (9) classifies the degree to which a patient’s functional capacity is limited as a result of angina symptoms. Ratings range from I to IV. Reliability and validity have been established (10).
Psychiatric Symptom Index
The Psychiatric Symptom Index (11) is a 29-item measure of psychological status. Patients rate the frequency, over the past week, with which they have experienced various psychological symptoms relevant to four clinical syndromes: depression, anxiety, anger and cognitive disturbance. The scale has excellent internal consistency (alpha coefficient 0.91), as well as strong validity.
Medical assessment
MPI studies with SPECT were electrocardiogram (ECG)-gated, and were used as the principal indicator of the presence or absence of myocardial ischemia, because the results of these tests have been shown to be more sensitive and specific in the diagnosis of CAD in men and women compared with standard ECG results (1). The images were reviewed by one of three trained nuclear imaging cardiologists who made the decision based on clinical impression.
The exercise stress tests were performed on a treadmill using a Bruce protocol, which entails four phases of increasing difficulty (speed and inclination), each lasting 3 min. The protocol was ceased when one of the following situations occurred: the patient reported considerable pain, the patient was out of breath, the patient presented with significant anomalies on the ECG (eg, greater than 3 mm ST segment deviation) or the patient achieved at least 90% of the maximal heart rate expected for their age. Peak heart rate, and systolic and diastolic blood pressure achieved during exercise testing are reported in Table 2. The metabolic estimated cost (METS) achieved during exercise as well as its duration were also noted for each patient.
Statistical analysis
Variable preselection for entry into the model
Separate χ2 analyses were performed using MPI results (negative or positive, indicating absence or presence of myocardial ischemia, respectively) as the dependent variable. The predictor variables were the pain (location, description and type) or symptom variables. These analyses were performed to preselect variables to enter in a later backward, logistic regression equation. Given the large number of variables, only variables reaching P<0.20 were retained for later analyses.
Other pertinent clinical variables (eg, history of hypertension) were identified for men and women and forced into the backward logistic regression analyses if their univariate correlation with the dependent variable reached P<0.20.
Backward, logistical regression analyses
Separate analyses were performed for men and women.
Men
Based on the preliminary analyses, the following clinical variables were selected for entry into the final backward, logistic analysis: age (forced in); history of hypertension (forced in); resting systolic blood pressure (forced in); history of diabetes (forced in); history of CAD (forced in), type of daily angina pain (typical, atypical, nonspecific; forced in), time of testing (morning or evening; forced in); and number of METS achieved during exercise stress testing (forced in). The selected pain and symptom variables were: pain in zones 5, 11 and 25 of the DPM; perspiration, dizziness, numb feeling in shoulder or arm, numb feeling in chest area, sweaty hands; pain described as hot or burning, aching or heavy; and total number of nonpain symptoms reported.
Women
The control variables included: age (forced in); history of hypertension (forced in); history of diabetes (forced in); history of CAD events (forced in); type of daily angina pain (typical, atypical and nonspecific; forced in); number of METS achieved during exercise stress testing (forced in); and family history of diabetes (forced in). The selected pain and symptom variables were: headache, dizziness, numb feeling in face or neck, numb feeling in chest area, muscle tension, sweaty hands, tightness in the chest; pain described as throbbing, hot or burning, fearful, suffocating or pressing; total number of pain descriptors used; and total pain intensity.
The present analysis was performed to assess whether pain and other symptoms obtained in a standardized manner detect the presence of ischemia during exercise stress testing with MPI when controlling for pertinent clinical variables. Except for control variables, only predictor variables that reached P<0.05 were kept in the final model.
RESULTS
One hundred forty-two men and 157 women complained of chest pain during exercise stress testing. The prevalence of ischemia during testing, as detected by SPECT, was 66% in men (n=94; mean age=61 years) and 24% in women (n=38; mean age=62 years). Complete data were obtained for 127 men and 146 women.
Univariate analyses
Numerous predictor variables reached P<0.20 and were included in the final backward, logistic regression analysis (see analysis section above for full list). The following preliminary results were particularly noteworthy because they reached or approached significance.
Men
Absence of numb feeling in shoulder or arm (P=0.071), absence of sweaty hands (P=0.000), absence of chest pain described as ‘hot or burning’ (P=0.027), presence of chest pain described as ‘aching’ (P=0.052) and presence of pain described as ‘heavy’ (P=0.038), were all associated with a greater risk of concomitant ischemia during exercise testing.
Two other variables reaching P<0.107 were excluded from the backward, logistic regressions due to the fact that too few men reported them. More specifically, pain in the right facial region (DPM zone 1) and pain in the right middle back (DPM zone 26) were each reported by five men. In each case, ischemia was present.
Women
Absence of a headache (P=0.035), presence of numb feeling in neck or throat (P=0.004), presence of numb feeling in chest (P=0.06), presence of tightness in chest (P=0.096), presence of pain described as hot or burning (P=0.079), presence of pain described as fearful (P=0.043) and greater pain intensity (P=0.063) were all associated with a greater risk of concomitant ischemia during exercise testing.
Backward, logistic regression analyses
Men
Table 3 presents the results of the logistical regression analysis for men. Of the control variables, only the period of testing was significantly associated with presence of ischemia during testing (morning tests associated with greater risk of ischemia). The symptom variables contributing significantly to the prediction of ischemia were absence of sweaty hands, absence of chest pain described as ‘hot or burning’ and presence of chest pain described as ‘heavy’.
TABLE 3.
Factors contributing to the prediction of ischemia in men
| Variables entered | P | OR | 95% CI |
|---|---|---|---|
| Block 1 (forced in) | |||
| Age | 0.353 | 1.024 | 0.974 to 1.075 |
| History of coronary events or procedures | 0.197 | 1.750 | 0.749 to 4.089 |
| Lifetime diagnosis of hypertension | 0.978 | 1.012 | 0.432 to 2.369 |
| Baseline systolic blood pressure | 0.052 | 1.023 | 1.000 to 1.048 |
| Diabetes | 0.323 | 1.872 | 0.540 to 6.493 |
| Type of daily angina pain | 0.088 | 0.700 | 0.464 to 1.055 |
| Time of day tested | 0.034 | 0.401 | 0.173 to 0.931 |
| METS achieved during testing | 0.259 | 0.852 | 0.645 to 1.125 |
| Model χ2=23.543 | 0.003 | ||
| Block 2 | |||
| Sweaty hands | 0.025 | 0.125 | 0.020 to 0.768 |
| Chest pain described as hot or burning | 0.035 | 0.364 | 0.142 to 0.932 |
| Chest pain described as heavy | 0.039 | 4.579 | 1.08 to 19.408 |
| Block χ2=15.222 | 0.002 | ||
| Full model χ2=38.765 | 0.000 | ||
METS Metabolic estimated cost
The addition of these symptoms improved accuracy of prediction only slightly beyond that which was furnished by the control variables. Overall accuracy increased from 72% to 77%, the specificity increased from 40% to 56%, while sensitivity remained the same at 88%.
Women
The results of the logistical regression analysis for women are presented in Table 4. Women with a history of coronary events or procedures experienced more ischemia than women with no such history. Women with a family history of diabetes were more likely to experience ischemia during testing compared with those without. Ischemic women achieved fewer METS during testing. In addition, women who experienced pain that was more typical of angina during the past month were more likely to develop ischemia during testing compared with women with nonspecific pain. The symptom factors contributing significantly to the prediction of ischemia were absence of headache, numb feeling in the face or neck, numb feeling in the chest area, muscle tension and chest pain described as hot or burning.
TABLE 4.
Factors contributing to the prediction of exercise stress ischemia in women
| Variables entered | P | OR | 95% CI |
|---|---|---|---|
| Block 1 (forced in) | |||
| Age | 0.037 | 1.06 | 1.003 to 1.119 |
| History of coronary events | 0.057 | 2.805 | 0.971 to 8.104 |
| Lifetime diagnosis of hypertension | 0.048 | 0.398 | 0.159 to 0.992 |
| Lifetime diagnosis of diabetes | 0.158 | 0.269 | 0.044 to 1.662 |
| Family history of diabetes | 0.007 | 3.585 | 1.424 to 9.029 |
| Type of daily angina pain | |||
| TP versus AP | 0.019 | 6.197 | 1.343 to 28.592 |
| TP versus NSP | 0.047 | 6.728 | 1.022 to 44.306 |
| METS achieved during testing | 0.160 | 0.783 | 0.557 to 1.102 |
| Model χ2=33.652 | 0.000 | ||
| Block 2 | |||
| Headache | 0.001 | 0.040 | 0.006 to 0.245 |
| Numb feeling in the face or neck | 0.048 | 4.544 | 1.014 to 20.371 |
| Numb feeling in the chest | 0.003 | 14.575 | 2.42 to 87.771 |
| Muscle tension | 0.013 | 5.197 | 1.409 to 19.165 |
| Chest pain described as hot or burning | 0.014 | 4.338 | 1.348 to 13.962 |
| Block χ2=37.169 | 0.000 | ||
| Full model χ2=70.820 | 0.000 | ||
AP Atypical pain; METS Metabolic estimated cost; NSP Nonspecific pain; TP Typical pain
The addition of these symptoms improved our capacity to detect who experienced ischemia. Sensitivity climbed from 32% (control variables only) to 70%, while specificity dropped slightly from 94% to 92%. Total accuracy increased from 78% to 86%.
Secondary analyses
To understand why detection of ischemia from symptoms was so disappointing in this sample of men, we examined the capacity of chest pain (all locations confounded) to predict ischemia in the overall sample of men recruited for the study (ie, those with and without angina). The presence of chest pain increased the likelihood of observing ischemia compared with no chest pain (OR 1.6; P=0.029). Thus, in men undergoing elective exercise stress testing, the presence of chest pain may be so predictive of ischemia that seeking a more refined evaluation of this pain may not be particularly helpful.
DISCUSSION
The hypothesis that ‘atypical’ or nonpain symptoms would be helpful in improving detection of ischemia in women, but not men, was supported in this prospective study. Symptoms added little to the sensitivity of the detection model in men. The presence of atypical symptoms (sweaty hands and chest pain described as ‘hot or burning’) in men was actually inversely related to the presence of ischemia. Milner et al (12) obtained similar results in patients with ACS. On the other hand, men who described their chest pain as ‘heavy’ had an almost fivefold chance of experiencing ischemia compared with men who did not use this descriptive. Further, while pain in the right middle back area (zone 26) was reported by only five men in the current study, it was associated with ischemia in each case, suggesting that this site may be highly indicative of ischemia when present. Pain in the right anterior shoulder (zone 5) showed a similar trend. That pain experienced in the back or right shoulder areas may be important is corroborated by previous findings in men presenting to the ED with non-traumatic chest pain (2). In the latter study, pain in the right shoulder (zones 5 and 23 on the DPM), retrosternal pain (zone 6), and pain in the right middle back (zone 26) contributed significantly to the prediction of ischemia on follow-up testing.
In women, examination of the symptom profile proved particularly informative, and improved sensitivity of the model from 32% to 70%. Chest pain described as hot or burning was associated with a fourfold greater risk of simultaneously experiencing ischemia during exercise stress testing. Milner et al (12) had similarly reported that chest pain was associated with a positive risk for ACS in women. Nonpain symptoms further contributed to the detection of myocardial ischemia in the current study. Women who experienced numb feelings in the face or neck or in the chest area were five to 15 times more likely to exhibit ischemia compared with women without these symptoms. A complaint of generalized muscle tension beginning during or immediately following exercise stress testing was similarly associated with a greater risk of ischemia. While clinicians commonly question as to the presence of chest, arm or neck pain during their evaluation of possible CAD, the current results strongly suggest the importance of examining other nonpainful sensations as well. Clearly, however, sensations in the neck and chest area, whether painful or not, seem particularly important in women.
Interestingly, despite common reports of symptoms such as nausea, indigestion, fatigue and palpitations in women with ACS or exercise-induced ischemia (3), no significant association between these symptoms and ischemia emerged in the current study, nor in that of Milner et al (12) in patients with suspected ACS.
Other investigations have obtained some divergent results. For example, in the Multicenter Chest Pain Study, Cunningham et al (13) reviewed the medical records of 7734 patients who had presented to the ED with chest pain. Of four symptoms examined, diaphoresis was associated with a greater risk for acute MI in both men and women. Zucker et al (14) also used a chart review process and examined five symptoms of ACS in 10,525 patients presenting to the ED with chest pain or other symptoms suggestive of acute cardiac ischemia. In addition to chest pain, nausea and vomiting predicted the presence of acute cardiac ischemia in men and women, whereas dizziness was a negative predictor. These studies differed in many respects from the current investigation: sheer number of patients, type of patients (unstable versus stable disease processes), design (retrospective from chart review versus prospectively with standardized questionnaires), number of symptoms evaluated (few versus in depth evaluation of type, intensity and location), variations in geographic settings, and inclusion of racial or ethnic minorities. Such differences may partly explain some of the observed contradictions in findings.
Finally, our study has a few limitations worthy of mention. While we recruited a large sample of men and women, the overall number of patients, particularly women, who experienced ischemia with concomitant chest pain was surprisingly low. The analyses may have lacked sufficient power to detect other, weaker predictors of ischemia. Given the small sample size, it is also possible that our multivariable models are over-fit. As such, our findings may be more appropriately considered as hypothesis generating at this time. Moreover, the participants in both samples were mainly French speaking and Caucasian, and attended a cardiac tertiary hospital, where the prevalence of CAD is higher than in other institutions. In our opinion, this should not influence the relationships observed between somatic complaints and ischemia. Nonetheless, further research in other ethnic groups would be helpful in assessing the generalizability of these results beyond Caucasians. Finally, only patients with concomitant chest pain were included in the current analyses to more closely resemble patients presenting to the ED with a chief complaint of nontraumatic chest pain. However, it may be that other symptoms, such as nausea, palpitations and weakness, may be more important in the diagnosis of silent (nonpainful) ischemia in women or men. This will be the subject of a future report.
CONCLUSION
Our results suggest that both painful and nonpainful sensations, particularly in the neck and chest area, are strongly related to exercise-induced ischemia in women undergoing elective testing for stable CAD. Further research will be needed to validate the usefulness of these symptom predictors in an independent group of women with potential CAD, whether stable or unstable.
Meanwhile, it may be confusing for the clinician to appreciate what is most helpful in the diagnosis of ischemia in men and women; results obtained from descriptive analyses that reveal a large number of sex differences in symptom experience in patients with ACS or ischemia, or from analyses that have sought to evaluate the value of these symptoms in predicting presence of ischemic events. More research is needed before we can successfully answer this question. DeVon and Zerwic (3) caution practitioners to consider the totality of symptoms reported by patients. Most patients experience chest pain. In men, this general symptom may be sufficiently useful in diagnosing potential CAD, as suggested by our study. However, in women, it is the associated symptoms or the more specific aspects of the pain that may complete the picture. If the clinician is aware of the symptoms associated with myocardial perfusion defects, then this increases the chances of making a rapid diagnosis. Indeed, inclusion of these associated symptoms markedly increased sensitivity of detection in women in the current study.
ACKNOWLEDGEMENT
The authors thank Karine St-Jean (MA) for her help in the recruitment and coordination of the study.
Footnotes
SUPPORT: This study was supported in part by the Canadian Institutes of Health Research (CIHR, MOP 53242) and by the Heart and Stroke Foundation of Canada (HSFC).
REFERENCES
- 1.Swahn E. The care of patients with ischaemic heart disease from a gender perspective. Eur Heart J. 1998;19:1758–65. doi: 10.1053/euhj.1998.1205. [DOI] [PubMed] [Google Scholar]
- 2.D’Antono B, Dupuis G, Fleet R, Marchand A, Burelle D. Sex differences in chest pain and prediction of exercise-induced ischemia. Can J Cardiol. 2003;19:515–22. [PubMed] [Google Scholar]
- 3.DeVon HA, Zerwic JJ. Symptoms of acute coronary syndromes: Are there gender differences? A review of the literature. Heart Lung. 2002;31:235–45. doi: 10.1067/mhl.2002.126105. [DOI] [PubMed] [Google Scholar]
- 4.D’Antono B, Dupuis G, Fortin C, Arsenault A, Burelle D. Angina symptoms in men and women with stable coronary artery disease and evidence of exercise-induced myocardial perfusion defects. Am Heart J. doi: 10.1016/j.ahj.2005.06.028. (In press) [DOI] [PubMed] [Google Scholar]
- 5.Fleet RP, Dupuis G, Marchand A, Burelle D, Beitman BD. Detecting panic disorder in emergency department chest pain patients: A validated model to improve recognition. Ann Behav Med. 1997;19:124–31. doi: 10.1007/BF02883329. [DOI] [PubMed] [Google Scholar]
- 6.Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24:57–65. doi: 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 8.Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1:277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 9.Campeau L. Grading of angina pectoris. Circulation. 1976;54:522–3. [PubMed] [Google Scholar]
- 10.Cox J, Naylor CD. The Canadian Cardiovascular Society grading scale for angina pectoris: Is it time for refinements? Ann Intern Med. 1992;117:667–83. doi: 10.7326/0003-4819-117-8-677. [DOI] [PubMed] [Google Scholar]
- 11.Ilfeld FW. Further validation of a psychiatric symptom index in a normal population. Psychol Rep. 1976;39:1215–28. [Google Scholar]
- 12.Milner KA, Funk M, Arnold A, Vaccarino V. Typical symptoms are predictive of acute coronary syndromes in women. Am Heart J. 2002;143:283–8. doi: 10.1067/mhj.2002.119759. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham MA, Lee TH, Cook EF, et al. The effect of gender on the probability of myocardial infarction among emergency department patients with acute chest pain: A report from the Multicenter Chest Pain Study Group. J Gen Intern Med. 1989;4:392–8. doi: 10.1007/BF02599688. [DOI] [PubMed] [Google Scholar]
- 14.Zucker Dr, Griffith JL, Beshansky JR, Selker HP. Presentations of acute myocardial infarction in men and women. J Gen Intern Med. 1997;12:79–87. doi: 10.1046/j.1525-1497.1997.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]