Abstract
A 60-year-old man, known for stable coronary artery disease, was admitted for suspected unstable angina. In the previous month, the patient presented with progressive dyspnea on light exertion. In the preceding four months, he had experience occasional episodes of flushing and diarrhea, and had inexplicably lost 22.7 kg. Night sweats and fever were absent. ST segment elevation in the inferior leads and ST segment depression in the precordial leads were documented during an episode of chest pain. The coronary angiogram showed diffuse disease with 70% stenosis of the left anterior descending coronary artery and 50% stenosis on the second diagonal (D2). An echocardiogram showed a patent foramen ovale. Balloon angioplasty and stenting were performed on the two lesions. Two days later, prolonged chest pain recurred. Cardiac catheterization was repeated and showed occlusive thrombus within the stent on the D2. Angioplasty was repeated. Symptoms recurred 36 h later, with the electrocardiogram showing ST segment elevation. The first angiogram was reviewed and vasospasm was suspected on a branch of the D2, on the second marginal and in the distal circumflex artery. The diagnosis of vasospastic angina was retained. Beta-blockers were replaced by high doses of a calcium channel blocker with an excellent clinical response. The case described is of a patient with an acute coronary syndrome, vasospastic angina, in-stent thrombosis and carcinoid disease. Coronary vasospasm was attributed to serotonin, which was secreted by the carcinoid tumour that reached an atherosclerotic coronary vasculature through a patent foramen ovale, thereby avoiding pulmonary inactivation.
Keywords: Acute coronary syndrome, Carcinoid tumour, Serotonin, Spasms
Abstract
Un homme de 60 ans, atteint d’une coronaropathie stable connue, a été hospitalisé en raison d’une présomption d’angine instable. Depuis un mois, il souffrait de dyspnée progressive à l’effort léger. Depuis quatre mois, il avait aussi subi des épisodes occasionnels de diarrhée et de bouffées vasomotrices et avait perdu du poids de manière inexpliquée (22,7 kg). Il n’avait ni sueurs nocturnes ni fièvre. Une surélévation du segment ST dans les dérivations inférieures et une dépression du segment ST dans les dérivations précordiales ont été documentées pendant un épisode de douleurs thoraciques. Un cathétérisme cardiaque a révélé une maladie diffuse comportant 70 % de sténose dans l’artère interventriculaire antérieure et 50 % de sténose dans la deuxième diagonale (D2). L’échocardiogramme a révélé une communication interauriculaire manifeste. Une angioplastie par ballonnet avec endoprothèse a été effectuée dans les deux lésions. Deux jours plus tard, les douleurs thoraciques prolongées reprenaient. On a fait un nouveau cathétérisme cardiaque qui a démontré un thrombus occlusif en D2. L’angioplastie a été reprise. Les symptômes se sont manifestés de nouveau 36 heures plus tard, et l’électrocardiogramme a révélé une surélévation du segment ST. Après révision du premier cathétérisme, on a fortement présumé la présence de vasospasmes dans une branche de D2, dans la deuxième artère marginale et dans l’artère auriculoventriculaire. Le diagnostic d’angine angiospastique a alors été retenu. Les bétabloquants ont été remplacés par de fortes doses d’inhibiteur calcique, qui ont obtenu une excellence réponse clinique. Ce cas fait donc état d’un patient souffrant d’un syndrome coronarien aigu, d’angine angiospastique, de thrombose dans l’endoprothèse et d’une maladie carcinoïde. Le vasospasme coronaire était attribué à la sérotonine sécrétée par la tumeur carcinoïde qui atteignait le réseau vasculaire coronarien athéroscléreux par une artère auriculoventriculaire, évitant ainsi l’inactivation pulmonaire.
A 60-year-old man with a seven-year history of coronary artery disease was admitted for suspected unstable angina. He was well until two weeks previously, when he experienced several episodes of squeezing chest pain while at rest, lasting 5 min to 15 min, accompanied by dyspnea and sweating. Episodes were relieved by sublingual nitroglycerine. In the previous month, the patient had also noted progressive dyspnea on light exertion and new-onset nocturnal dyspnea, with recent episodes of flushing and diarrhea. He had unaccountably lost 22.7 kg in the preceding four months. Night sweats and fever were absent. Recently, furosemide 40 mg had been added to control new-onset lower limb edema. Other daily medications were acetylsalicylic acid 81 mg, valsartan 80 mg and lovastatin 20 mg.
In the emergency room, the physical examination showed a mildly obese man without distress. Central venous pressure was elevated with V waves. A grade II/VI holosystolic regurgitant murmur was heard at the left and right sternal borders. Lung auscultation was normal, and a 2 cm pitting edema was noted at the ankles with stasis dermatitis. The electrocardiogram (ECG) was normal and cardiac troponin was negative. The patient was admitted with a diagnosis of acute coronary syndrome. The patient was started on heparin, nitrates and a beta-blocker. Echocardiography showed normal left ventricular systolic function with grade 2 mitral and grade 3 tricuspid regurgitation. The sonographer also reported abnormal hepatic lesions compatible with liver metastases.
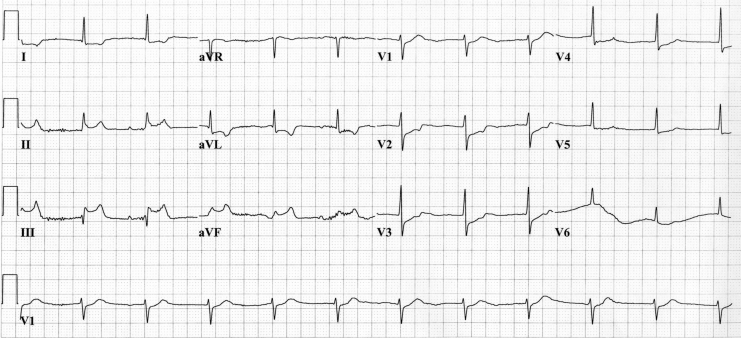
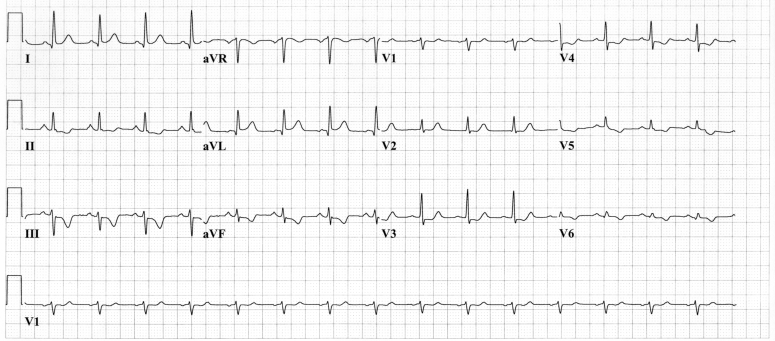
During his hospital stay, the patient presented with several episodes of chest pain at rest. The ECG showed a junctional rhythm with ST segment elevation in the inferior leads (II, III and aVF) and ST segment depression in the other leads (Figure 1). Coronary angiography showed diffuse disease with 70% stenosis in the mid segment of the left anterior descending coronary artery and a 50% stenosis on the second diagonal branch (D2). Balloon angioplasty was performed and stents were deployed at these two sites. Two days after the procedure, prolonged chest pain recurred while the patient was at rest. ST segment depression was noted in V3 to V6, with a T wave inversion in the inferolateral leads (Figure 2). Coronary angiography was performed again and occlusive thrombus was noted within the stent deployed in the D2. Angioplasty was performed successfully within this lesion and the ECG returned to normal. This episode was associated with myocardial necrosis. The patient’s troponin I concentration rose to 36.4 μg/L (normal 0.5 μg/L or less) and creatine kinase-MB concentration rose to 21.6 U/L (normal 10 U/L or less).
Figure 1.
The initial electrocardiogram during episodes of chest pain at rest
Figure 2.
Electrocardiogram during episodes of recurrent, prolonged chest pain at rest
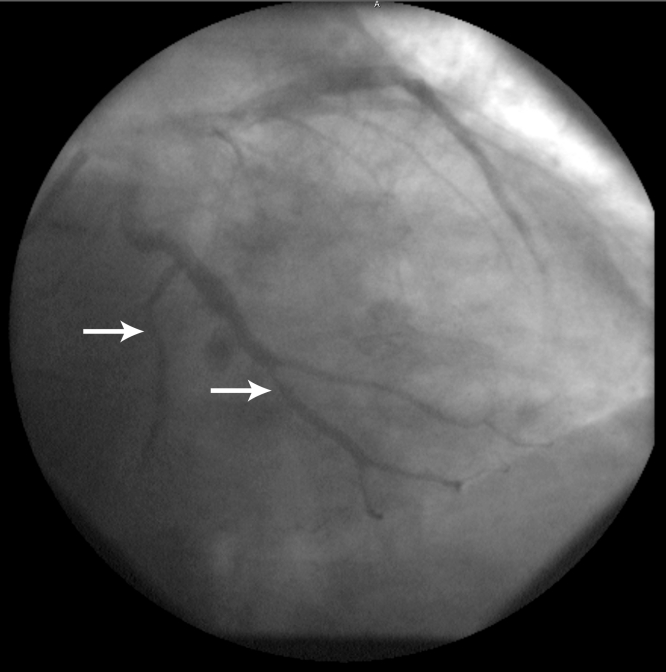
Thirty-six hours later, symptoms recurred, with the ECG showing ST segment re-elevation in the inferior leads and ST segment depression in the other leads, as in the first ECG (Figure 1). The first coronary angiogram was then reviewed and vasospasm was suspected on a branch of the D2, in the second marginal and in the distal circumflex artery (Figure 3). The diagnosis of vasospastic angina was retained and the beta-blocker therapy was replaced by diltiazem 360 mg daily. The patient’s symptoms did not recur. Investigation of the hepatic lesions confirmed cancer of unknown origin. A computed tomography scan of the thorax was normal and did not show lymph node enlargement. A liver biopsy confirmed the diagnosis of a carcinoid tumour. The urinary metabolite, 5-hydroxyindole acetic acid (5-HIAA) was elevated to 1179 μmol/24 h (normal value less than 50 μmol/24 h). Octreotide therapy was started.
Figure 3.
Left coronary angiogram showing spasm of the mid-circumflex (bottom arrow) and second obtuse marginal branch (top arrow)
Several weeks after discharge, the patient consulted with progressive dyspnea without chest pain. Transthoracic echocardiography was performed, followed by transesophageal echocardiography. Severe tricuspid (grade 4 of 4) and pulmonary (grade 4 of 4) regurgitation with important valve thickening, reduced mobility and noncoaptation of the cups was found. The left atrium and ventricle sizes were normal, as were their function. A patent foramen oval was demonstrated with a significant right to left shunt. These features were compatible with carcinoid heart disease.
DISCUSSION
Carcinoid heart disease is a feature of a rare, paraneoplastic syndrome. The carcinoid syndrome is classically characterized by secretory diarrhea, flushing, bronchoconstriction and hemodynamic instability (1,2). The overall incidence of carcinoid tumours in the United States is estimated at one to two cases per 100,000 people (3). Multiple substances released by the tumours (serotonin, calcitonin, gastrin, histamine, dopamine, substance P, corticotropin, growth hormone, neurotensin, prostaglandins and kallikrein) have been proposed to explain heart structural damages and hemodynamic derangements (1,3). The specific clinical syndrome is a function of the type of tumour and its secretory products; the most common are serotonin and bradykinin. Approximately 20% of carcinoid disease presents with symptoms of heart failure (4). Carcinoid heart lesions are characterized by plaque-like and fibrous endocardial thickenings, which may cause retraction and fixation of the leaflets of the tricuspid and pulmonary valves. Tricuspid regurgitation is characteristic, but tricuspid stenosis, pulmonary regurgitation and pulmonary stenosis may also occur (3,5,6). Endocardial involvement suggests an advanced disease process (1,3,6).
In the present case, the preponderance of lesions on the right side of the heart suggested that carcinoid heart disease was related to the factors secreted into the hepatic vein by the liver metastases in contrast with patients with primary ovarian carcinoid tumours. Because the venous drainage of the ovary bypasses the portal circulation, carcinoid heart disease may develop in the absence of liver metastasis (7). The valves and endocardium of the right side of the heart are classically involved because lung tissue inactivates vasoactive humoral substances, thus sparing the left heart. Left-sided valvular pathology occurs in less than 10% of patients with cardiac involvement and is mostly associated with a right-to-left shunt, or a primary bronchial carcinoid or carcinoid metastases to the lungs (5,8). These abnormalities allow serotonin-rich venous blood to enter the left heart chambers without passing through the pulmonary capillaries (1,6). Patients with heart disease have two- to fourfold higher levels of serotonin in their serum and 5-HIAA in their urine than other patients with the carcinoid syndrome (3). This supports the role of serotonin in carcinoid heart disease, because progression of the disease and severity of cardiac involvement appear to be proportional to the urinary levels of 5-HIAA (1,2,9). Serotonin is a naturally occurring vasoactive substance that has diverse cardiophysiological effects. It may either constrict or dilate blood vessels depending on the vessel site and the state of the endothelium. The vasoconstrictive action is believed to be mediated by 5-hydroxytryptamine-2 serotoninergic receptors on platelets, endothelium and vascular smooth muscle cells. Serotonin amplifies the release and augments the action of several other vasoconstricting mediators including histamine, angiotensin II, prostaglandin F2-alpha and noradrenaline. Vascular dilation by serotonin is mediated by 5-hydroxytryptamine-1 receptors and nitric oxide when endothelial function is preserved, and the predominant physiological effect of serotonin is vasodilation. When endothelial function is abnormal, such as in coronary atherosclerosis, the 5-hydroxytryptamine-2 receptor will be relatively unopposed because of the loss of 5-hydroxytryptamine-1 dilator activity, and the predominant effect of serotonin becomes vasoconstriction (10–12). The vascular effects of serotonin are so varied that it can induce different contractile responses in separate segments of the same coronary artery (13,14).
Coronary artery spasm is an unusual manifestation of the carcinoid syndrome. The pathogenesis of serotonin in coronary vasospasm is difficult to document because of rapid metabolism by adsorption onto circulating platelets, or by endothelial or neural reuptake (15). Nevertheless, the half-life of exogenously administered serotonin was calculated to be 1.2 min (16). This allows time for serotonin released from the tumour to reach the coronary artery tree through the patent foramen ovale. To our knowledge, only three cases of presumptive coronary spasm have been reported in the literature; all were characterized by ST segment elevation followed by ventricular arrhythmias and cardiac arrest (17–19). While one was triggered by maximal exercise testing in a patient with metastatic carcinoid disease in whom nonsignificant coronary artery disease was documented (18), one was triggered during the investigation of an exophytic tumour with laser bronchoscopy (17); in the other patient, the coronary spasm occurred during an episode of flushing (19). We believe that our case of coronary spasm during angiography is the first to be documented. The ST elevation in the inferior leads during chest pain was presumably secondary to the spasm of the right coronary artery, because there were no obstructive lesions in the right coronary artery on coronary angiography. Indeed, spasm of the nondominant circumflex artery (Figure 3) does not appear sufficient to account for the ST elevation in the inferior leads. Moreover, the junctional rhythm is probably secondary to sinoatrial node ischemia in the setting of right coronary artery vasospasm.
As in classic vasospastic angina, symptoms are usually well controlled with a calcium channel blocker. Although valvular disease was secondary to carcinoid disease, it is plausible that the vasospasm in our patient was due to the release of the vasoactive molecules into the coronary circulation via the right-to-left shunt. Indeed, increased preload of the right ventricle secondary to severe valvular disease could have facilitated the shunt. The beneficial clinical response to the high dose of calcium channel blocker, as reported previously (18), also supports this hypothesis. Interestingly, the instent thrombosis could have been favoured by high systemic serotonin levels, because serotonin is a powerful activator of platelets (20,21) and intravenous injection of serotonin stimulates arterial thrombus formation (22).
CONCLUSION
We report an unusual presentation of acute coronary syndrome in a patient with coronary artery and carcinoid heart disease. This was characterized by protean manifestations such as right-sided valvular disease with right-to-left shunting of serotonin through a patent foramen ovale favouring coronary vasospasm and in-stent thrombosis.
REFERENCES
- 1.Anderson AS, Krauss D, Lang R. Cardiovascular complications of malignant carcinoid disease. Am Heart J. 1997;134:693–702. doi: 10.1016/s0002-8703(97)70053-x. [DOI] [PubMed] [Google Scholar]
- 2.Robiolio PA, Rigolin VH, Wilson JS, et al. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92:790–5. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- 3.Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999;340:858–68. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 4.Kvols LK. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Ann N Y Acad Sci. 1994;733:464–70. doi: 10.1111/j.1749-6632.1994.tb17296.x. [DOI] [PubMed] [Google Scholar]
- 5.Pellikka PA, Tajik AJ, Khandheria BK, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87:1188–96. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 6.Roberts WC. A unique heart disease associated with a unique cancer: Carcinoid heart disease. Am J Cardiol. 1997;80:251–6. doi: 10.1016/s0002-9149(97)00340-8. [DOI] [PubMed] [Google Scholar]
- 7.Chaowalit N, Connolly HM, Schaff HV, Webb MJ, Pellikka PA. Carcinoid heart disease associated with primary ovarian carcinoid tumor. Am J Cardiol. 2004;93:1314–5. doi: 10.1016/j.amjcard.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 8.Levine RJ, Folse R. Hemodynamic observations in a case of carcinoid heart disease associated with an atrial right-to-left shunt. Am Heart J. 1961;62:830–4. doi: 10.1016/0002-8703(61)90670-6. [DOI] [PubMed] [Google Scholar]
- 9.Moller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348:1005–15. doi: 10.1056/NEJMoa021451. [DOI] [PubMed] [Google Scholar]
- 10.Frishman WH, Grewall P. Serotonin and the heart. Ann Med. 2000;32:195–209. doi: 10.3109/07853890008998827. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuoka W, Egashira S, Tagawa H, et al. Augmentation of coronary responsiveness to serotonin at the site of X-ray-induced intimal thickening in miniature pigs. Cardiovasc Res. 1995;30:246–54. [PubMed] [Google Scholar]
- 12.Lamping KG, Dole WP. Acute hypertension selectively potentiates constrictor responses of large coronary arteries to serotonin by altering endothelial function in vivo. Circ Res. 1987;61:904–13. doi: 10.1161/01.res.61.6.904. [DOI] [PubMed] [Google Scholar]
- 13.Myers JH, Mecca TE, Webb RC. Direct and sensitizing effects of serotonin agonists and antagonists on vascular smooth muscle. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S44–8. doi: 10.1097/00005344-198500077-00014. [DOI] [PubMed] [Google Scholar]
- 14.McFadden EP, Clarke JG, Davies GJ, Kaski JC, Haider AW, Maseri A. Effect of intracoronary serotonin on coronary vessels in patients with stable angina and patients with variant angina. N Engl J Med. 1991;324:648–54. doi: 10.1056/NEJM199103073241002. [DOI] [PubMed] [Google Scholar]
- 15.Vanhoutte PM, Cohen RA. The elusory role of serotonin in vascular function and disease. Biochem Pharmacol. 1983;32:3671–4. doi: 10.1016/0006-2952(83)90134-x. [DOI] [PubMed] [Google Scholar]
- 16.Zinner MJ, Kasher F, Jaffe BM. The hemodynamic effects of intravenous infusions of serotonin in conscious dogs. J Surg Res. 1983;34:171–8. doi: 10.1016/0022-4804(83)90057-4. [DOI] [PubMed] [Google Scholar]
- 17.Mehta AC, Rafanan AL, Bulkley R, Walsh M, DeBoer GE. Coronary spasm and cardiac arrest from carcinoid crisis during laser bronchoscopy. Chest. 1999;115:598–600. doi: 10.1378/chest.115.2.598. [DOI] [PubMed] [Google Scholar]
- 18.Topol EJ, Fortuin NJ. Coronary artery spasm and cardiac arrest in carcinoid heart disease. Am J Med. 1984;77:950–2. doi: 10.1016/0002-9343(84)90549-7. [DOI] [PubMed] [Google Scholar]
- 19.Petersen KG, Seemann WR, Plagwitz R, Kerp L. Evidence for coronary spasm during flushing in the carcinoid syndrome. Clin Cardiol. 1984;7:445–8. doi: 10.1002/clc.4960070805. [DOI] [PubMed] [Google Scholar]
- 20.Vikenes K, Farstad M, Nordrehaug JE. Serotonin is associated with coronary artery disease and cardiac events. Circulation. 1999;100:483–9. doi: 10.1161/01.cir.100.5.483. [DOI] [PubMed] [Google Scholar]
- 21.Ardlie NG, Cameron HA, Garrett J. Platelet activation by circulating levels of hormones: A possible link in coronary heart disease. Thromb Res. 1984;36:315–22. doi: 10.1016/0049-3848(84)90322-0. [DOI] [PubMed] [Google Scholar]
- 22.Belougne-Malfatti E, Aguejouf O, Doutremepuich F, Doutremepuich C. Action of neurotransmitters: Acetylcholine, serotonin, and adrenaline in an experimental arterial thrombosis induced by oxygen free radicals. Thromb Res. 1997;88:435–9. doi: 10.1016/s0049-3848(97)00281-8. [DOI] [PubMed] [Google Scholar]