Abstract
After a successful Fontan procedure, children and adolescents should improve their exercise capacity. However, several studies have shown that these children have a reduced maximal oxygen consumption compared with healthy children. The lower exercise performance in these patients was mainly explained by a reduced cardiorespiratory functional capacity. However, it has recently been reported that the lower exercise performance may also be related to altered skeletal muscle function. Moreover, exercise training had a beneficial impact on several parameters related to exercise tolerance in these patients. The main studies supporting these observations are reviewed, with a focus on the physiological adaptation and limitation of the exercise performance as well as the benefits of exercise training in patients after a Fontan procedure.
Keywords: Exercise, Fontan procedure, Skeletal muscle
Abstract
Après une l’intervention chirurgicale de type Fontan, les enfants et les adolescents devraient améliorer leur tolérance à l’effort. Cependant, plusieurs études démontrent la persistance d’une diminution de la consommation d’oxygène maximale après la chirurgie comparativement à celle des enfants sains. Cette tolérance limitée à l’effort s’explique par une réduction des fonctions cardiorespiratoires. Cependant, selon des données récentes, une atteinte fonctionnelle des muscles squelettiques contribue rait aussi à la tolérance limitée à l’effort. De plus, l’entraînement à l’exercice améliore plusieurs paramètres reliés à la tolérance à l’effort chez ces patients. Cette revue résume les principales études expliquant ces observations et particulièrement l’adaptation physiologique et les limites reliées à l’exercice et les effets d’un programme d’entraînement à l’exercice chez des patients ayant eu une intervention chirurgicale de type Fontan.
The Fontan procedure, originally described by Fontan and Baudet (1) in 1971, has been a major contribution in improving the quality of life and survival of children and adolescents with cyanotic congenital heart disease who were limited in their physical activities and at high risk of premature death (2). The primary indication of this procedure was to palliate tricuspid atresia. This congenital heart malformation, defined as an inadequate development of the tricuspid valve, is characterized by the absence of the tricuspid orifice, the presence of atrial and ventricular septal defects, and right ventricular hypoplasia. The Fontan procedure was developed to address this specific type of single ventricle physiology (1,2). The procedure was designed to deviate the venous return from the superior and inferior vena cavae through the pulmonary arteries, with the right atrium being used to propel the venous return through the lungs. Thus, partial restoration of the pulmonary blood flow was achieved, alleviating the mixed arterial and venous blood characteristic of this malformation (1). Several modifications of the Fontan procedure have been developed to address the single functional ventricle in general (3–9).
This procedure confers a significant survival benefit, relief of cyanosis and improvement of approximately 20% in exercise tolerance. In fact, patients have a 30% to 40% lower exercise tolerance than that of healthy, age-matched controls (10,11). Several limitations of the cardiorespiratory functions may explain this reduced exercise tolerance. However, these patients may benefit from exercise training, as is documented in other patients with congenital heart diseases (12). This benefit of improved exercise performance is of importance because life expectancy of patients following the Fontan procedure has increased significantly – 70% at five years and 60% at 10 years (13).
Patients who have had a Fontan procedure constitute an heterogeneous group. Indeed, there are significant hemodynamic differences between the different Fontan procedures (for example, a classic Fontan procedure compared with an extracardiac Fontan procedure). A single left ventricle Fontan patient cannot be compared with a single right ventricle Fontan patient. In addition, a Fontan procedure performed for pulmonary atresia with an intact ventricular septum is different from a hypoplastic left heart in regard to pulmonary artery flow dynamics, coronary perfusion and morphology of the systemic ventricle. Although the population of patients with a Fontan procedure is heterogeneous, they share similar physiological and metabolic adaptations during exercise, and most likely share similar benefits from exercise training. The objective of the present report is to review the main studies on exercise physiology and training in these patients.
THE IMPACT OF THE FONTAN PROCEDURE ON EXERCISE TOLERANCE
In theory, the Fontan procedure may enhance exercise tolerance by reducing and/or eliminating the right-to-left shunt, thus decreasing the systemic ventricular volume overload. Comparing the exercise tolerance in patients before and after their Fontan procedure, Zellers et al (11) observed a 19% increase (22 mL/kg/min to 27 mL/kg/min) in maximal oxygen uptake (VO2max) in a cohort of 20 patients, while Driscoll et al (10), who studied 81 patients before and 27 patients after a Fontan procedure, noted a 16% increase (20.5 mL/kg/min to 24.3 mL/kg/min) in VO2max. In general, following the Fontan procedure, patients have a VO2max ranging from 15 mL/kg/min to 29 mL/kg/min (approximately 43% to 78% of normal VO2max) (14,15). Nevertheless, the long-term impact of this procedure on exercise tolerance is equivocal. Some may increase their exercise tolerance during the six years following surgery (16), while other may have a deterioration in their exercise tolerance (17). Deconditioning may contribute to this deterioration because aerobic exercise training has been shown to improve these patients’ functional capacity (18–21). Nevertheless, this long-term decline in exercise tolerance following the procedure may also be related to the disease progression (17) or to the reduced capacity to increase the cardiac output (Q) in response to exercise secondary to the Fontan-type circulation, thus limiting the oxygen transport to the periphery (22,23).
Slower oxygen uptake kinetics, ie, the precise integration of the cardiorespiratory system required to transport the oxygen from the atmosphere through active muscles to achieve oxidative phosphorylation (24), is well documented in patients with coronary artery disease, cyanotic congenital heart disease or heart failure (25–31). In patients who have had a Fontan procedure, slower oxygen uptake kinetics was observed compared with those of healthy individuals, suggesting alterations in the central component (oxygen transport) and/or in the peripheral component (oxygen utilization) during exercise (32). Notwithstanding the benefit of the Fontan procedure, there is evidence that a significant reduction in exercise tolerance persists in these patients (Table 1). The main factors related to the lower exercise capacity may be due to cardiovascular function, cardiac dysrhythmia, ventilatory and pulmonary functions, and the skeletal muscle status and function.
TABLE 1.
Comparison of physiological parameters in patients before and after the Fontan procedure
| Parameters | Authors (reference) |
|---|---|
| Higher or no change in maximal oxygen consumption | Driscoll et al (10), Zellers et al (11), Driscoll et al (41) |
| Lower or no change in maximal heart rate | Driscoll et al (10), Zellers et al (11), Driscoll et al (41) |
| No change in submaximal heart rate | Driscoll et al (41) |
| Higher or no change in blood pressure at exercise | Driscoll et al (10), Zellers et al (diastolic blood pressure) (11) |
| Abnormal cardiac output at exercise | Driscoll et al (10), Zellers et al (11), Nir et al (16) |
| Higher arterial blood saturation at rest | Driscoll et al (10), Zellers et al (11), Driscoll et al (41) |
| Higher arterial blood saturation at exercise | Driscoll et al (10), Zellers et al (11), Driscoll et al (41) |
| Higher or no change in arrhythmias | Driscoll et al (10), Driscoll et al (41) |
| Lower or no change in ventilatory equivalent for oxygen | Zellers et al (11), Driscoll et al (41) |
| No change in maximum minute ventilation | Driscoll et al (10) |
CARDIOVASCULAR FUNCTION
According to the Fick principle (oxygen uptake = flow × arteriovenous difference in oxygen) (24), parameters from the central and peripheral components may be implicated in the exercise intolerance observed in patients after a Fontan procedure. Regarding the central component, an abnormal Q response to exercise is present in patients after a Fontan procedure (Table 2). This alteration may be explained by a reduced stroke volume at rest (10,33) with an insufficient stroke volume response to exercise (33), and lower resting and exercise ejection fractions (34). In fact, the change in the patients’ physiology as a result of the Fontan procedure does not allow the Q to adequately increase in response to exercise, as it would in healthy individuals. Because the pulmonary blood flow is not driven by the right ventricle, it is reduced and almost nonpulsatile (22,23). This leads to a limited increase in pulmonary blood flow in response to exercise, a reduced left ventricle filling and a smaller stroke volume, resulting in a reduced increase in Q. The blood pressure response to exercise seems normal and adapted to the workload (14,15). However, a higher diastolic blood pressure response during exercise has also been reported (11), and may be explained by the absence of a systemic-to-pulmonary shunt (11) and/or may be linked to the abnormal reflex signals coming from the ergoreceptors (15). A lower peak systolic blood pressure, probably due to a lower Q, has also been documented (35). Finally, a reduced maximal heart rate has been observed in some (Table 2) but not all patients (36). In some instances, a similar (33) or 15% higher heart rate (37) has been reported in Fontan patients during submaximal exercise compared with controls. These altered chronotropic responses to exercise may be explained by a sinus node dysfunction following surgery or concomitant abnormal autonomic heart rate control (10,11,35,38).
TABLE 2.
Comparison of physiological parameters in patients who underwent the Fontan procedure compared with healthy individuals
| Parameters | Author (reference) |
|---|---|
| Lower maximal oxygen consumption | Brassard et al (15), Nir et al (16), Mocellin and Gildein (32), Ohuchi et al (35), Rhodes et al (36), Grant et al (37), Durongpisitkul et al (39), Troutman et al (42), Chua et al (43), Inai et al (44), Fredriksen et al (45), Rosenthal et al (72), Weipert et al (73), Zajac et al (74), Ohuchi et al (75) |
| No change in cardiac output at exercise | Grant et al (37) |
| Lower maximal heart rate | Brassard et al (15), Harrison et al (34), Ohuchi et al (35), Durongpisitkul et al (39), Troutman et al (42), Chua et al (43), Inai et al (44), Fredriksen et al (45), Rosenthal et al (72), Weipert et al (73), Zajac et al (74), Ohuchi et al (75) |
| Lower arterial blood saturation at rest | Nir et al (16), Brassard et al (15), Gewillig et al (33) |
| No change in maximal heart rate | Grant et al (37), Ohuchi et al (75) |
| No change in arterial blood saturation at rest | Grant et al (37), Inai et al (44), Zajac et al (74), Ohuchi et al (75) |
| Higher submaximal heart rate | Grant et al (37) |
| Lower arterial blood saturation maximum | Brassard et al (15), Grant et al (37), Durongpisitkul et al (39), Troutman et al (42), Chua et al (43), Inai et al (44), Fredriksen et al (45), Zajac et al (74) |
| No change in submaximal heart rate | Gewillig et al (33) |
| Higher maximal ventilatory equivalent for carbon dioxide | Brassard et al (15), Grant et al (37), Troutman et al (42), Chua et al (43), Inai et al (44) |
| No change in blood pressure at rest | Brassard et al (15), Nir et al (16), Gewillig et al (33), Chua et al (systolic blood pressure) (43), Inai et al (44), Zajac et al (74), Ohuchi et al (systolic blood pressure) (75) |
| No change in maximal ventilatory equivalent for carbon dioxide | Ohuchi et al (75) |
| Higher maximal ventilatory equivalent for oxygen | Brassard et al (15), Grant et al (37) |
| Lower blood pressure at exercise | Nir et al (systolic blood pressure) (16), Gewillig et al (33), Ohuchi et al (systolic blood pressure) (35), Inai et al (44), Ohuchi et al (systolic blood pressure) (75) |
| No change in maximal ventilatory equivalent for oxygen | Durongpisitkul et al (39) |
| No change in blood pressure at exercise | Nir et al (diastolic blood pressure) (16), Chua et al (systolic blood pressure) (43), Zajac et al (74) |
| Lower maximal minute ventilation | Harrison et al (34), Grant et al (37), Fredriksen et al (45), Zajac et al (74), Ohuchi et al (75) |
| Lower cardiac output at exercise | Durongpisitkul et al (39) |
CARDIAC ARRHYTHMIAS
Cardiac arrhythmias are frequent following the Fontan procedure, particularly at the supraventricular level (13). Stretching of the right atrium, decreased ventricular function and atrioventricular valve insufficiency have all been associated with cardiac arrhythmias (13). In fact, 20% of these patients need an antiarrhythmic agent and/or a pacemaker after a mean follow-up of five years (13). Among 59 patients, Durongpisitkul et al (39) reported that 10 patients presented premature ventricular contractions, one patient had ventricular tachycardia, one patient had atrial fibrillation and eight patients had nonspecific ST-T wave changes. However, all these arrhythmias occurred during exercise only, and none were documented during the recovery period (39). Also, first-degree atrioventricular block, junctional rhythm at rest, premature ventricular contractions and premature atrial complexes during exercise have been observed (40).
VENTILATORY AND PULMONARY FUNCTIONS
Before surgery, Fontan patients have an increased ventilatory drive at rest and during exercise. This is demonstrated by an elevated ventilatory equivalent for oxygen at rest and at maximal exercise (14). Following the surgical procedure, the ventilatory equivalent for oxygen may be corrected at rest but stays slightly elevated at maximal exercise (15,37). This is the consequence of an increased physiological dead space to tidal volume ratio resulting from ventilation or perfusion mismatch and a persistent right-to-left shunt, hypoxic stimulation of ventilation and/or the need to maintain acid-base homeostasis by eliminating carbon dioxide from a reduced blood volume (10,14,39,41). Furthermore, a significant increment of the ventilatory equivalent for carbon dioxide has also been reported (15,37,42–44), suggesting excessive ventilation in patients after a Fontan procedure compared with healthy individuals. These abnormalities may be secondary to the increased physiological dead space to tidal volume ratio or a lower chemoreceptors threshold for arterial carbon dioxide partial pressure (42).
Patients, after a Fontan procedure, have a reduced forced vital capacity and forced expiratory volume in 1 s compared with predicted values (45). A lower forced vital capacity, forced expiratory volume in 1 s, vital capacity, total lung capacity and diffusion capacity to carbon monoxide has been noted compared with healthy individuals (15,40,46). Diaphragmatic palsy following the surgical intervention, respiratory muscle weakness or restricted chest wall may be responsible for the patient’s reduced pulmonary function. However, no significant correlation was found between pulmonary function and exercise tolerance (15,40), suggesting that pulmonary function is probably not a limiting factor to exercise tolerance in these patients.
Arterial blood saturation returned almost to normal following the Fontan procedure; however, it stays slightly lower than that of healthy individuals and decreases with exercise (Table 2). This reduction may be the consequence of an intra-pulmonary right-to-left shunt that may become more important over time following the procedure, coronary sinus blood return to the pulmonary venous atrium, an intracardiac right-to-left shunt, a significant physiological dead space and/or a ventilation/perfusion mismatch with an abnormal pulmonary blood flow distribution (10,14,17,37,47). The lower arterial blood saturation may also be the result of a flow increment in the lungs’ superior lobes with increased pulmonary vascular resistance (47).
A FORGOTTEN PLAYER: THE PERIPHERY
Until recently, the periphery, mainly characterized by the skeletal muscles, has never been the centre of attention in studies evaluating exercise tolerance in patients after a Fontan procedure. Several investigators have demonstrated a poor relationship between central hemodynamic measurements and exercise tolerance in patients with congestive heart failure (CHF) (48–51), a population that shares similar characteristics with patients after a Fontan procedure. The contribution of abnormal skeletal muscle function to the low exercise performance of patients with CHF is now well recognized (52,53). It was recently demonstrated that the reduced exercise performance in Fontan patients may be related in part to altered skeletal muscle hemodynamics during exercise, as well as endothelial dysfunction (44). Thus, these alterations may lead to skeletal muscle function abnormalities in these patients as in CHF patients. The authors also observed reduced skeletal muscle endurance in a group of seven Fontan patients compared with healthy individuals (15). Some skeletal muscle alterations encountered in CHF patients, like muscle atrophy and weakness, morphological changes and/or altered metabolic capacity, may explain these results. The authors also observed a significant relationship between skeletal muscle strength and exercise tolerance, suggesting that the skeletal muscle function may be a limiting factor of exercise tolerance in these patients (15).
Skeletal muscle function may be evaluated noninvasively by ergoreflex activity (54). This reflex originates from small afferent receptors located in the muscle that are sensitive to metabolites produced during contraction (54). Some parameters that are less influenced by the Fontan procedure, ie, ventilation and blood pressure, may be overstimulated by an altered skeletal muscle function, leading to early exercise termination due to dyspnea and fatigue.
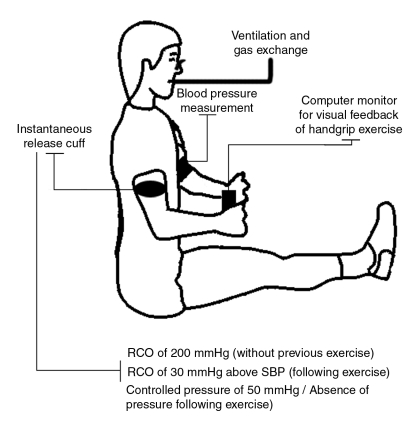
The method used to stimulate these ergoreceptors involves isolating their activity by trapping the metabolic milieu related to muscle work by a regional circulatory occlusion (RCO) (Figure 1) after muscular exercise until fatigue (55). Specifically, the evaluation of the ergoreflex activity included two randomly executed dynamic exercises. First, using the nondominant arm, patients are asked to contract dynamic handgrips with a hand dynamometer at 50% of their previously determined maximal voluntary contraction. This exercise is performed at a frequency of 30 handgrips/min to 40 handgrips/min until exhaustion. Ten seconds before the end of the exercise, an RCO of 30 mmHg above the resting systolic pressure is applied for 3 min with an instantaneous release cuff. Ventilation and blood pressure are taken during a 7 min period (3 min into RCO and 4 min after the RCO period). Secondly, after a 30 min rest, patients are asked to execute the same handgrip exercise, but the RCO period is replaced by a controlled pressure of 50 mmHg or by the absence of pressure for 3 min (15,55). Ventilation and blood pressure values are also recorded over the whole 7 min. The ergoreflex contribution to blood pressure is then quantified as the absolute values (value at the third minute of posthandgrip exercise with RCO minus value at the third minute of controlled posthandgrip) and as the percentage response to handgrip exercise followed by RCO compared with controlled posthandgrip (55). A controlled RCO of 200 mmHg without previous exercise was applied with the instantaneous release cuff first to ensure that exaggerated ergoreflex activation was not the result of the RCO per se.
Figure 1.
Schematic representation of the protocol used for the evaluation of the ergoreflex contribution. RCO Regional circulatory occlusion; SBP Systolic blood pressure
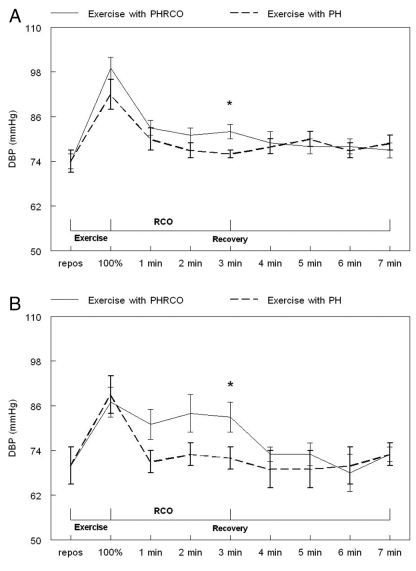
In healthy individuals, this ergoreflex activity may be a facilitative mechanism during exercise because it stimulates sympathetic drive, ventilation and vasoconstriction in the nonexercising limbs (56). In CHF patients, and possibly in Fontan patients, the excessive stimulation of ventilation and blood pressure by the ergoreceptors may have a deleterious impact on the cardiovascular and ventilatory systems (15,57). A higher ergoreflex contribution to blood pressure response was observed in seven Fontan patients (Figure 2) (15). These patients may thus rely earlier on anaerobic metabolism than healthy individuals, suggesting an abnormal oxidative capacity of their skeletal muscle function. Consequently, the overstimulation of the sympathetic drive and ventilation by this abnormal reflex activity may lead, with time, to an increased burden on the cardiorespiratory system and may favour the progression of the disease as seen in CHF (58).
Figure 2.
Comparison of diastolic blood pressure (DBP) in healthy individuals (A) and in Fontan patients (B) during the two handgrip repetitions with (solid lines) and without (dotted lines) regional circulatory occlusion (RCO). Data are presented as means ± SEMs. *P<0.05 posthandgrip controlled pressure (PH) versus posthandgrip regional circulatory occlusion (PHRCO). repos Rest
IMPACT OF EXERCISE TRAINING
It is well known that regular aerobic exercise training enhances the exercise tolerance in CHF patients (59–62). More specifically, the positive impacts of exercise training in these patients have been correlated to central and peripheral hemodynamics, ventilatory and metabolic responses, and the symptomatic status (63–66). Enhancements in the sympathetic response, autonomic heart rate control and variability have also been noticed (67). Furthermore, reduced exacerbated inflammatory responses leading to enhancement in exercise tolerance have also been observed following an exercise training program (68,69).
Because the contribution of altered skeletal muscle function to a reduced exercise capacity is well known in CHF patients (52), it is interesting to note that resistance exercise training leads to a positive impact on the periphery that may potentially enhance exercise tolerance. Specifically, an increase in muscular strength and endurance, a normalization of the ergoreflex feedback, and enhanced structural and metabolic characteristics of the skeletal muscle have all been observed in CHF patients (55,70,71). With these positive results of aerobic and resistance exercise training, it seems likely that such results may also be observed in Fontan patients.
Few studies have reported on the impact of exercise training in a limited number of patients after a Fontan procedure. Minamisawa et al (18) reported a 7% increase in maximal workload and VO2max and a 5% increase in exercise time in 11 patients following a 11- to 15-week aerobic exercise training program. Recently, Opocher et al (19) observed a 19% increase in both VO2max and oxygen pulse, and enhancement of submaximal heart rate and oxygen pulse pattern after an eight-month aerobic exercise training in 10 Fontan patients. At this point, an important issue needs to be raised regarding the positive impact of exercise training on exercise tolerance in Fontan patients in the previous two studies. In both, the positive influence of exercise training on VO2max was not compared with results of a control group to ensure that these observations were really the results of exercise training and not maturation of these young patients per se. In contrast, Brassard et al (15) did not observe any significant changes in VO2max in five Fontan patients following an eight-week aerobic and resistance exercise training program compared with a control group of four Fontan patients who did not exercise (15). However, a normalization of the ergoreflex contribution to blood pressure was noted, suggesting a possible reduction of the burden on the cardiovascular system (15). In addition, Balfour et al (20) observed a 6% increase in VO2max in a 19-year-old patient who was engaged in a three-month cardiac rehabilitation program. Finally, McCall and Humphrey (21) reported improvements in oximetry values, and exercise intensity and duration, as well as reduced fatigue perception in a young Fontan patient following a 22-week aerobic and muscular exercise training program before cardiac transplant.
Exercise training is thus an important feature in these young patients’ rehabilitation. In light of our results and the existing literature underlying the positive impact of aerobic and resistance exercise training in CHF patients, we can speculate that resistance exercise training involving the principal muscle groups needs to be added to an aerobic exercise training component to increase the benefit of training in Fontan patients. Because the single ventricle physiology precludes an increase in Q during exercise, the systemic oxygen transport is near its highest capacity with few possibilities to increase during aerobic exercise training. Accordingly, one must target the peripheral component (muscle) to enhance the ‘metabolic machinery’ and reduce the burden on the cardiovascular system.
CONCLUSIONS
Until recently, the explanation for the reduced exercise capacity in patients after a Fontan procedure was attributed to limited cardiorespiratory function. However, we already know that in patients with CHF, the skeletal muscle function seems to be another important component of exercise tolerance. This limited skeletal muscle function could be a major factor explaining the reduced exercise performance of patients after a Fontan procedure. Moreover, the impact of exercise training on VO2max in itself is small. However, further research is needed to better understand whether physical deconditioning is related to exercise intolerance and whether the patients need to be trained for a longer period of time to increase their exercise capacity. Interestingly, a significant impact of exercise training may be observed on skeletal muscle function, suggesting that this function may be implicated in the reduced exercise capacity in these patients as in patients with CHF. Most importantly, exercise training may have a positive influence on the quality of life in Fontan patients, even with a small amelioration in exercise capacity.
ACKNOWLEDGEMENTS
The Quebec Heart Institute Foundation supported the present work. Patrice Brassard is the recipient of a graduate research scholarship in pharmacy from the Rx & D Health Research Foundation Awards Program funded in partnership with the Canadian Institutes of Health Research. Paul Poirier is a clinician-scientist of the Fonds de la recherche en santé du Québec. The authors acknowledge the collaboration of Dr Christine Houde for her participation in the elaboration of the present manuscript, and thank Dr Gilles R Dagenais for his insightful review and comments regarding the present manuscript.
REFERENCES
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao PS. Tricuspid atresia. eMedicine Journal. 2001;2:1–49. [Google Scholar]
- 3.Bjork VO, Olin CL, Bjarke BB, Thoren CA. Right atrial-right ventricular anastomosis for correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1979;77:452–8. [PubMed] [Google Scholar]
- 4.Bridges ND, Lock JE, Castaneda AR. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation. 1990;82:1681–9. doi: 10.1161/01.cir.82.5.1681. [DOI] [PubMed] [Google Scholar]
- 5.Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation. 1990;82(5 Suppl):170–6. [PubMed] [Google Scholar]
- 6.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96:682–95. [PubMed] [Google Scholar]
- 7.Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66:613–21. [PubMed] [Google Scholar]
- 8.Marcelletti C, Corno A, Giannico S, Marino B. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J Thorac Cardiovasc Surg. 1990;100:228–32. [PubMed] [Google Scholar]
- 9.Waldman JD, Lamberti JJ, George L, et al. Experience with Damus procedure. Circulation. 1988;78:III32–9. [PubMed] [Google Scholar]
- 10.Driscoll DJ, Danielson GK, Puga FJ, Schaff HV, Heise CT, Staats BA. Exercise tolerance and cardiorespiratory response to exercise after the Fontan operation for tricuspid atresia or functional single ventricle. J Am Coll Cardiol. 1986;7:1087–94. doi: 10.1016/s0735-1097(86)80227-3. [DOI] [PubMed] [Google Scholar]
- 11.Zellers TM, Driscoll DJ, Mottram CD, Puga FJ, Schaff HV, Danielson GK. Exercise tolerance and cardiorespiratory response to exercise before and after the Fontan operation. Mayo Clin Proc. 1989;64:1489–97. doi: 10.1016/s0025-6196(12)65704-8. [DOI] [PubMed] [Google Scholar]
- 12.Therrien J, Fredriksen PM, Walker M, Granton J, Reid GJ, Webb G. A pilot study of exercise training in adult patients with repaired tetralogy of Fallot. Can J Cardiol. 2003;19:685–9. [PubMed] [Google Scholar]
- 13.Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five- to fifteen-year follow-up after Fontan operation. Circulation. 1992;85:469–96. doi: 10.1161/01.cir.85.2.469. [DOI] [PubMed] [Google Scholar]
- 14.Driscoll DJ, Durongpisitkul K. Exercise testing after the Fontan operation. Pediatr Cardiol. 1999;20:57–9. doi: 10.1007/s002469900397. [DOI] [PubMed] [Google Scholar]
- 15.Brassard P, Poirier P, Martin J, et al. Impact of exercise training on muscle function and ergoreflex in Fontan patients: A pilot study. Int J Cardiol. 2006;107:85–94. doi: 10.1016/j.ijcard.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Nir A, Driscoll DJ, Mottram CD, et al. Cardiorespiratory response to exercise after the Fontan operation: A serial study. J Am Coll Cardiol. 1993;22:216–20. doi: 10.1016/0735-1097(93)90837-q. (Erratum in 1993;22:1272) [DOI] [PubMed] [Google Scholar]
- 17.Reybrouck T, Rogers R, Weymans M, et al. Serial cardiorespiratory exercise testing in patients with congenital heart disease. Eur J Pediatr. 1995;154:801–6. doi: 10.1007/BF01959785. [DOI] [PubMed] [Google Scholar]
- 18.Minamisawa S, Nakazawa M, Momma K, Imai Y, Satomi G. Effect of aerobic training on exercise performance in patients after the Fontan operation. Am J Cardiol. 2001;88:695–8. doi: 10.1016/s0002-9149(01)01822-7. [DOI] [PubMed] [Google Scholar]
- 19.Opocher F, Varnier M, Sanders SP, et al. Effects of aerobic exercise training in children after the Fontan operation. Am J Cardiol. 2005;95:150–2. doi: 10.1016/j.amjcard.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 20.Balfour IC, Drimmer AM, Nouri S, Pennington DG, Hemkens CL, Harvey LL. Pediatric cardiac rehabilitation. Am J Dis Child. 1991;145:627–30. doi: 10.1001/archpedi.1991.02160060045018. [DOI] [PubMed] [Google Scholar]
- 21.McCall R, Humphrey R. Exercise training in a young adult late after a Fontan procedure to repair single ventricle physiology. J Cardiopulm Rehabil. 2001;21:227–30. doi: 10.1097/00008483-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Stromvall Larsson E, Eriksson BO. Haemodynamic adaptation during exercise in Fontan patients at a long-term follow-up. Scand Cardiovasc J. 2002;37:107–12. doi: 10.1080/4017430310001221. [DOI] [PubMed] [Google Scholar]
- 23.Cloutier A, Ash JM, Smallhorn JF, et al. Abnormal distribution of pulmonary blood flow after the Glenn shunt or Fontan procedure: Risk of development of arteriovenous fistulae. Circulation. 1985;72:471–9. doi: 10.1161/01.cir.72.3.471. [DOI] [PubMed] [Google Scholar]
- 24.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 3. Philadelphia: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 25.Belardinelli R, Zhang YY, Wasserman K, Purcaro A, Agostoni PG. A four-minute submaximal constant work rate exercise test to assess cardiovascular functional class in chronic heart failure. Am J Cardiol. 1998;81:1210–4. doi: 10.1016/s0002-9149(98)00093-9. [DOI] [PubMed] [Google Scholar]
- 26.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–9. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 27.Koike A, Hiroe M, Adachi H, et al. Oxygen uptake kinetics are determined by cardiac function at onset of exercise rather than peak exercise in patients with prior myocardial infarction. Circulation. 1994;90:2324–32. doi: 10.1161/01.cir.90.5.2324. [DOI] [PubMed] [Google Scholar]
- 28.Koike A, Yajima T, Adachi H, et al. Evaluation of exercise capacity using submaximal exercise at a constant work rate in patients with cardiovascular disease. Circulation. 1995;91:1719–24. doi: 10.1161/01.cir.91.6.1719. [DOI] [PubMed] [Google Scholar]
- 29.Sietsema KE, Cooper DM, Perloff JK, et al. Dynamics of oxygen uptake during exercise in adults with cyanotic congenital heart disease. Circulation. 1986;73:1137–44. doi: 10.1161/01.cir.73.6.1137. [DOI] [PubMed] [Google Scholar]
- 30.Sietsema KE, Ben-Dov I, Zhang YY, Sullivan C, Wasserman K. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest. 1994;105:1693–700. doi: 10.1378/chest.105.6.1693. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YY, Wasserman K, Sietsema KE, et al. O2 uptake kinetics in response to exercise. A measure of tissue anaerobiosis in heart failure. Chest. 1993;103:735–41. doi: 10.1378/chest.103.3.735. [DOI] [PubMed] [Google Scholar]
- 32.Mocellin R, Gildein P. Velocity of oxygen uptake response at the onset of exercise: A comparison between children after cardiac surgery and healthy boys. Pediatr Cardiol. 1999;20:17–20. doi: 10.1007/s002469900385. [DOI] [PubMed] [Google Scholar]
- 33.Gewillig MH, Lundstrom UR, Bull C, Wyse RK, Deanfield JE. Exercise responses in patients with congenital heart disease after Fontan repair: Patterns and determinants of performance. J Am Coll Cardiol. 1990;15:1424–32. doi: 10.1016/s0735-1097(10)80034-8. [DOI] [PubMed] [Google Scholar]
- 34.Harrison DA, Liu P, Walters JE, et al. Cardiopulmonary function in adult patients late after Fontan repair. J Am Coll Cardiol. 1995;26:1016–21. doi: 10.1016/0735-1097(95)00242-7. [DOI] [PubMed] [Google Scholar]
- 35.Ohuchi H, Hasegawa S, Yasuda K, Yamada O, Ono Y, Echiga S. Severely impaired cardiac autonomic nervous activity after the Fontan operation. Circulation. 2001;104:1513–8. doi: 10.1161/hc3801.096326. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes J, Garofano RP, Bowman FO, Jr, Grant GP, Bierman FZ, Gersony WM. Effect of right ventricular anatomy on the cardiopulmonary response to exercise. Implications for the Fontan procedure. Circulation. 1990;81:1811–7. doi: 10.1161/01.cir.81.6.1811. [DOI] [PubMed] [Google Scholar]
- 37.Grant GP, Mansell AL, Garofano RP, Hayes CJ, Bowman FO, Jr, Gersony WM. Cardiorespiratory response to exercise after the Fontan procedure for tricuspid atresia. Pediatr Res. 1988;24:1–5. doi: 10.1203/00006450-198807000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Rydberg A, Rask P, Hornsten R, Teien D. Heart rate variability in children with Fontan circulation. Pediatr Cardiol. 2004;25:365–9. doi: 10.1007/s00246-003-0552-x. [DOI] [PubMed] [Google Scholar]
- 39.Durongpisitkul K, Driscoll DJ, Mahoney DW, et al. Cardiorespiratory response to exercise after modified Fontan operation: Determinants of performance. J Am Coll Cardiol. 1997;29:785–90. doi: 10.1016/s0735-1097(96)00568-2. [DOI] [PubMed] [Google Scholar]
- 40.Mahle WT, Wernovsky G, Bridges ND, Linton AB, Paridon SM. Impact of early ventricular unloading on exercise performance in preadolescents with single ventricle Fontan physiology. J Am Coll Cardiol. 1999;34:1637–43. doi: 10.1016/s0735-1097(99)00392-7. [DOI] [PubMed] [Google Scholar]
- 41.Driscoll DJ, Feldt RH, Mottram CD, Puga FJ, Schaff HV, Danielson GK. Cardiorespiratory response to exercise after definitive repair of univentricular atrioventricular connection. Int J Cardiol. 1987;17:73–81. doi: 10.1016/0167-5273(87)90034-9. [DOI] [PubMed] [Google Scholar]
- 42.Troutman WB, Barstow TJ, Galindo AJ, Cooper DM. Abnormal dynamic cardiorespiratory responses to exercise in pediatric patients after Fontan procedure. J Am Coll Cardiol. 1998;31:668–73. doi: 10.1016/s0735-1097(97)00545-7. [DOI] [PubMed] [Google Scholar]
- 43.Chua TP, Iserin L, Somerville J, Coats AJ. Effects of chronic hypoxemia on chemosensitivity in patients with univentricular heart. J Am Coll Cardiol. 1997;30:1827–34. doi: 10.1016/s0735-1097(97)00360-4. [DOI] [PubMed] [Google Scholar]
- 44.Inai K, Saita Y, Takeda S, Nakazawa M, Kimura H. Skeletal muscle hemodynamics and endothelial function in patients after Fontan operation. Am J Cardiol. 2004;93:792–7. doi: 10.1016/j.amjcard.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 45.Fredriksen PM, Therrien J, Veldtman G, et al. Lung function and aerobic capacity in adult patients following modified Fontan procedure. Heart. 2001;85:295–9. doi: 10.1136/heart.85.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsson ES, Eriksson BO, Sixt R. Decreased lung function and exercise capacity in Fontan patients. A long-term follow-up. Scand Cardiovasc J. 2003;37:58–63. doi: 10.1080/14017430310007045. [DOI] [PubMed] [Google Scholar]
- 47.Matsushita T, Matsuda H, Ogawa M, et al. Assessment of the intrapulmonary ventilation-perfusion distribution after the Fontan procedure for complex cardiac anomalies: Relation to pulmonary hemodynamics. J Am Coll Cardiol. 1990;15:842–8. doi: 10.1016/0735-1097(90)90284-v. [DOI] [PubMed] [Google Scholar]
- 48.Higginbotham MB, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51:52–60. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 49.Franciosa JA, Ziesche S, Wilen M. Functional capacity of patients with chronic left ventricular failure. Relationship of bicycle exercise performance to clinical and hemodynamic characterization. Am J Med. 1979;67:460–6. doi: 10.1016/0002-9343(79)90794-0. [DOI] [PubMed] [Google Scholar]
- 50.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol. 1981;47:33–9. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 51.Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1985;55:1037–42. doi: 10.1016/0002-9149(85)90742-8. [DOI] [PubMed] [Google Scholar]
- 52.Jobin J, Doyon JF. Peripheral muscle limitations to exercise in patients with congestive heart failure: Implications for rehabilitation. In: Jobin J, Maltais F, LeBlanc, Simard C, editors. Advances in Cardiopulmonary Rehabilitation. Champaign: Human Kinetics; 2000. pp. 90–104. [Google Scholar]
- 53.Maltais F, Débigaré R, Saey D, Jobin J. Peripheral muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: Two diseases, one common consequence. In: Jobin J, Maltais F, LeBlanc, Simard C, editors. Advancing the Frontiers of Cardiopulmonary Rehabilitation. Champaign: Human Kinetics; 2002. pp. 181–92. [Google Scholar]
- 54.Piepoli M, Clark AL, Coats AJ. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol. 1995;269:H1428–36. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- 55.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: Effects of physical training. Circulation. 1996;93:940–52. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 56.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324–30. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 57.Floras JS. Clinical aspects of sympathetic activation and parsympathetic withdrawal in heart failure. J Am Coll Cardiol. 1993;22(Suppl A):72A–84A. doi: 10.1016/0735-1097(93)90466-e. [DOI] [PubMed] [Google Scholar]
- 58.Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J. 1999;137:1050–6. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 59.Appleton B. The role of exercise training in patients with chronic heart failure. Br J Nurs. 2004;13:452–6. doi: 10.12968/bjon.2004.13.8.12780. [DOI] [PubMed] [Google Scholar]
- 60.Lloyd-Williams F, Mair FS, Leitner M. Exercise training and heart failure: A systematic review of current evidence. Br J Gen Pract. 2002;52:47–55. [PMC free article] [PubMed] [Google Scholar]
- 61.McKelvie RS, Teo KK, McCartney N, Humen D, Montague T, Yusuf S. Effects of exercise training in patients with congestive heart failure: A critical review. J Am Coll Cardiol. 1995;25:789–96. doi: 10.1016/0735-1097(94)00428-S. [DOI] [PubMed] [Google Scholar]
- 62.Adamopoulos S, Parissis JT, Kremastinos DT. New aspects for the role of physical training in the management of patients with chronic heart failure. Int J Cardiol. 2003;90:1–14. doi: 10.1016/s0167-5273(02)00504-1. [DOI] [PubMed] [Google Scholar]
- 63.Conn EH, Williams RS, Wallace AG. Exercise responses before and after physical conditioning in patients with severely depressed left ventricular function. Am J Cardiol. 1982;49:296–300. doi: 10.1016/0002-9149(82)90504-5. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988;78:506–15. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with chronic heart failure delays ventilatory anaerobic threshold and improves submaximal exercise performance. Circulation. 1989;79:324–9. doi: 10.1161/01.cir.79.2.324. [DOI] [PubMed] [Google Scholar]
- 66.Coats AJ, Adamopoulos S, Meyer TE, Conway J, Sleight P. Effects of physical training in chronic heart failure. Lancet. 1990;335:63–6. doi: 10.1016/0140-6736(90)90536-e. [DOI] [PubMed] [Google Scholar]
- 67.Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–31. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 68.Adamopoulos S, Parissis J, Kroupis C, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–7. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 69.Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–63. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 70.Kiilavuori K, Naveri H, Salmi T, Harkonen M. The effect of physical training on skeletal muscle in patients with chronic heart failure. Eur J Heart Fail. 2000;2:53–63. doi: 10.1016/s1388-9842(00)00058-1. [DOI] [PubMed] [Google Scholar]
- 71.Coats AJ. Exercise and heart failure. Cardiol Clin. 2001;19:517–24. doi: 10.1016/s0733-8651(05)70233-2. [DOI] [PubMed] [Google Scholar]
- 72.Rosenthal M, Bush A, Deanfield J, Redington A. Comparison of cardiopulmonary adaptation during exercise in children after the atriopulmonary and total cavopulmonary connection Fontan procedures. Circulation. 1995;91:372–8. doi: 10.1161/01.cir.91.2.372. [DOI] [PubMed] [Google Scholar]
- 73.Weipert J, Koch W, Haehnel JC, Meisner H. Exercise capacity and mid-term survival in patients with tricuspid atresia and complex congenital cardiac malformations after modified Fontan-operation. Eur J Cardiothorac Surg. 1997;12:574–80. doi: 10.1016/s1010-7940(97)00220-0. [DOI] [PubMed] [Google Scholar]
- 74.Zajac A, Tomkiewicz L, Podolec P, Tracz W, Malec E. Cardiorespiratory response to exercise in children after modified fontan operation. Scand Cardiovasc J. 2002;36:80–5. doi: 10.1080/140174302753675348. [DOI] [PubMed] [Google Scholar]
- 75.Ohuchi H, Arakaki Y, Hiraumi Y, Tasato H, Kamiya T. Cardiorespiratory response during exercise in patients with cyanotic congenital heart disease with and without a Fontan operation and in patients with congestive heart failure. Int J Cardiol. 1998;66:241–51. doi: 10.1016/s0167-5273(98)00249-6. [DOI] [PubMed] [Google Scholar]