Abstract
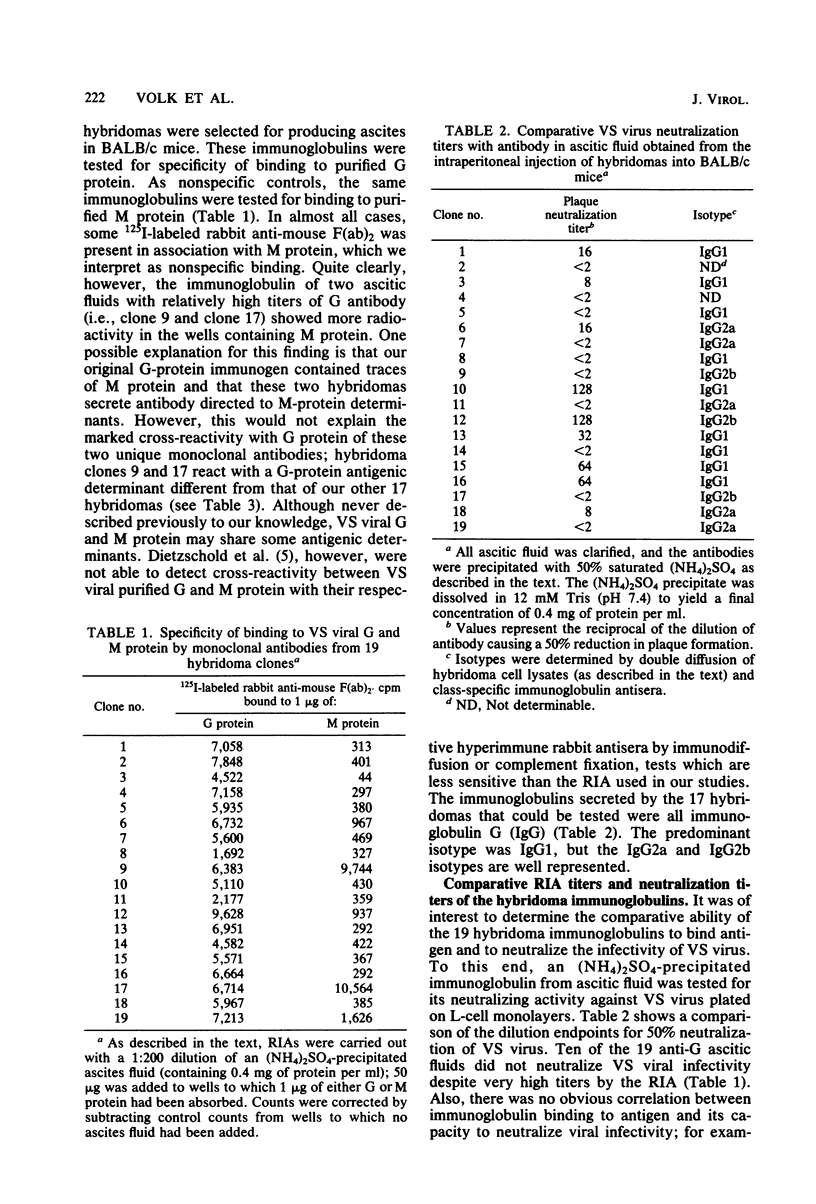
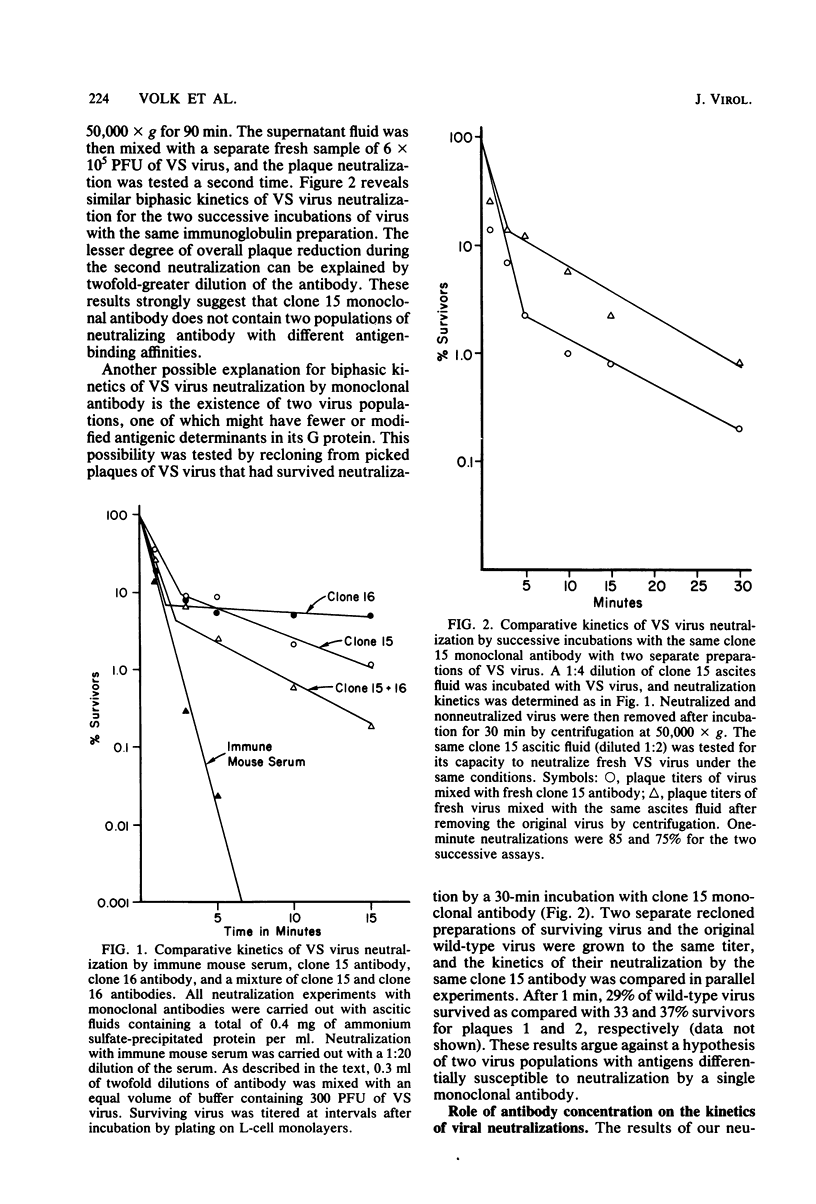
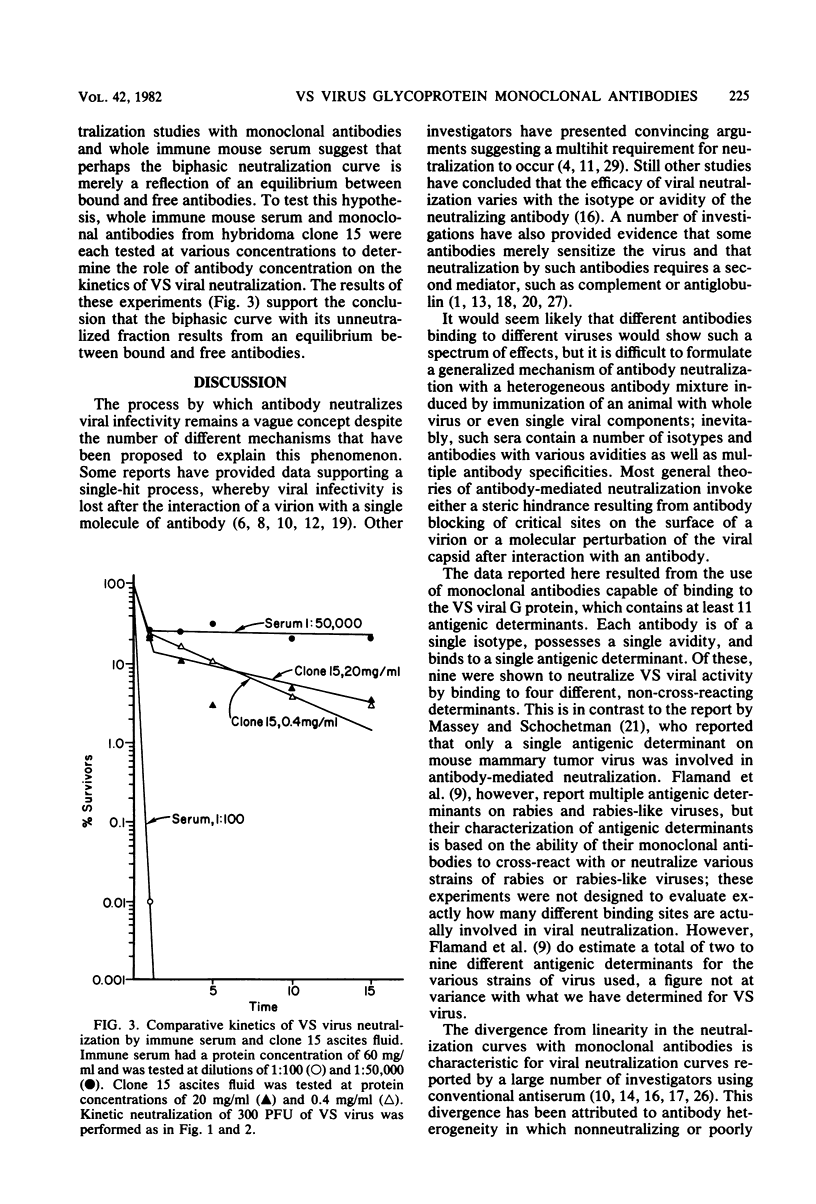
Nineteen independently isolated hybridomas producing monoclonal antibodies to the glycoprotein of vesicular stomatitis virus were isolated and studied for their capacity to neutralize viral infectivity. By measuring competitive binding of 125I-labeled monoclonal antibodies in a radioimmunoassay. 11 different, non-cross-reacting antigenic determinants were identified on the vesicular stomatitis virus G protein. All monoclonal antibodies reacting with determinants 1, 2, 3, and 4 resulted in viral neutralization, whereas those binding to the other seven determinants did not neutralize infectivity. The mixture of two monoclonal antibodies binding to different determinants resulted in a more rapid neutralization than either antibody alone, suggesting that different antibodies can exert a synergistic effect on viral neutralization. Kinetic experiments revealed biphasic neutralization curves similar to those expected for heterologous antibody. No evidence could be obtained to relate biphasic kinetics of viral neutralization to heterogeneous populations either of antibody molecules or of virus. The possible significance of the kinetic data with monoclonal antibodies is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe W. K., Notkins A. L. Neutralization of an infectious herpes simplex virus-antibody complex by anti-gamma-globulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):447–451. doi: 10.1073/pnas.56.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Repik P., Obijeski J. F., Moore N. F., Wagner R. R. Restitution of infectivity to spikeless vesicular stomatitis virus by solubilized viral components. J Virol. 1975 Jul;16(1):75–84. doi: 10.1128/jvi.16.1.75-84.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972 Sep;16(3):391–398. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Della-Porta A. J., Westaway E. G. A multi-hit model for the neutralization of animal viruses. J Gen Virol. 1978 Jan;38(1):1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Schneider L. G., Cox J. H. Serological characterization of the three major proteins of vesicular stomatitis virus. J Virol. 1974 Jul;14(1):1–7. doi: 10.1128/jvi.14.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Flamand A., Wiktor T. J., Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. II. The glycoprotein. J Gen Virol. 1980 May;48(1):105–109. doi: 10.1099/0022-1317-48-1-105. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. THE INTERACTION OF NEWCASTLE DISEASE VIRUS AND NEUTRALIZING ANTIBODY. Virology. 1965 Jan;25:38–47. doi: 10.1016/0042-6822(65)90249-7. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Dowdle W. R. Immunological basis of the adenovirus 8-9 cross-reaction. J Virol. 1970 Dec;6(6):782–787. doi: 10.1128/jvi.6.6.782-787.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick C. L., Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry. 1972 Mar;9(3):325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- Huggett D. O., Rodríguez J. E., McKee A. P. Infectious antibody-reovirus complexes. Infect Immun. 1972 Dec;6(6):996–1002. doi: 10.1128/iai.6.6.996-1002.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFFERTY K. J. THE INTERACTION BETWEEN VIRUS AND ANTIBODY. I. KINETIC STUDIES. Virology. 1963 Sep;21:61–75. doi: 10.1016/0042-6822(63)90305-2. [DOI] [PubMed] [Google Scholar]
- Lewenton-Kriss S., Mandel B. Studies on the nonneutralizable fraction of poliovirus. Virology. 1972 Jun;48(3):666–678. doi: 10.1016/0042-6822(72)90151-1. [DOI] [PubMed] [Google Scholar]
- MANDEL B. Studies on the interactions of poliomyelitis virus, antibody, and host cells in tissue culture system. Virology. 1958 Oct;6(2):424–447. doi: 10.1016/0042-6822(58)90092-8. [DOI] [PubMed] [Google Scholar]
- Majer M. Virus sensitization. Curr Top Microbiol Immunol. 1972;58:69–84. doi: 10.1007/978-3-642-65357-5_2. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of poliovirus: a hypothesis to explain the mechanism and the one-hit character of the neutralization reaction. Virology. 1976 Feb;69(2):500–510. doi: 10.1016/0042-6822(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Viral epitopes and monoclonal antibodies: isolation of blocking antibodies that inhibit virus neutralization. Science. 1981 Jul 24;213(4506):447–449. doi: 10.1126/science.6264601. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Lipid composition of purified vesicular stomatitis viruses. J Virol. 1971 Jan;7(1):59–70. doi: 10.1128/jvi.7.1.59-70.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Wagner R. R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979 Jun 10;254(11):4313–4316. [PubMed] [Google Scholar]
- Radwan A. I., Burger D. The role of sensitizing antibody in the neutralization of equine arteritis virus by complement or anti-IgG serum. Virology. 1973 Jun;53(2):366–371. doi: 10.1016/0042-6822(73)90215-8. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. The neutralization of arboviruses. II. Neutralization in heterologous virus-serum mixtures with four group B arboviruses. Virology. 1965 Aug;26(4):528–537. doi: 10.1016/0042-6822(65)90314-4. [DOI] [PubMed] [Google Scholar]



