Abstract
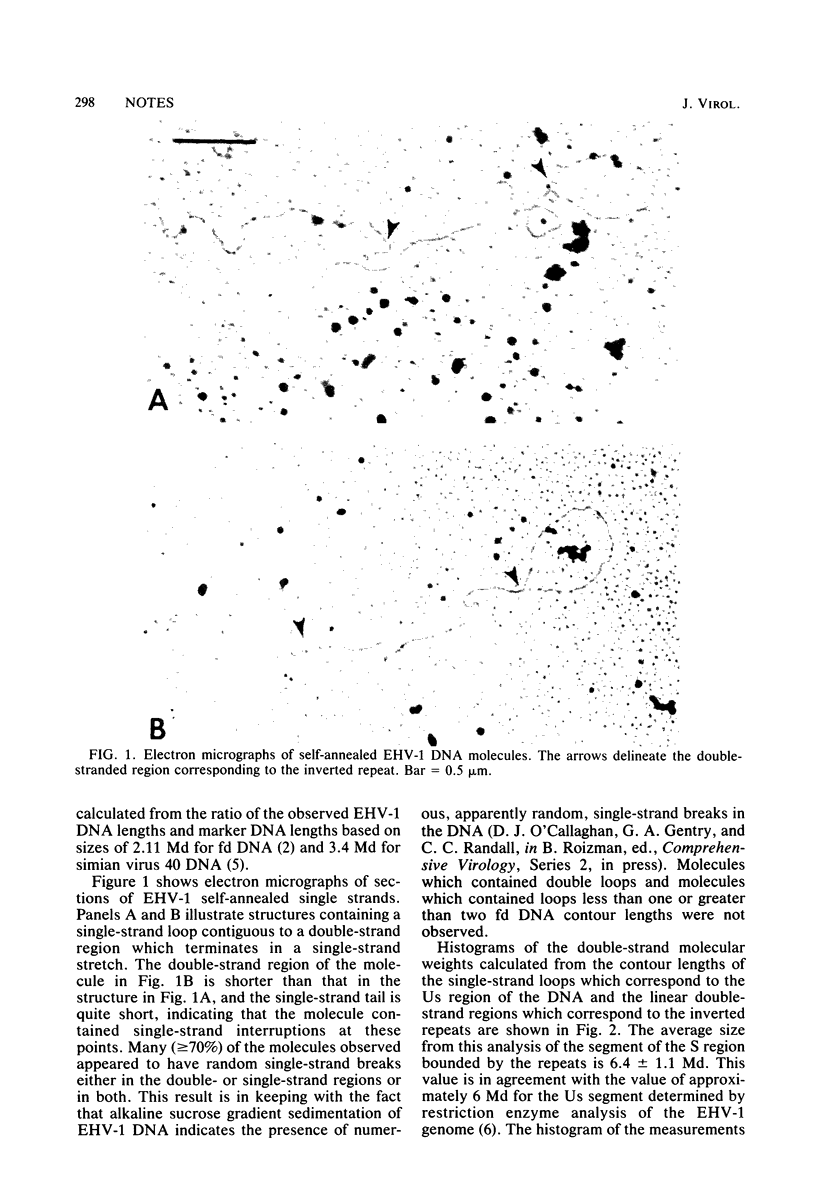
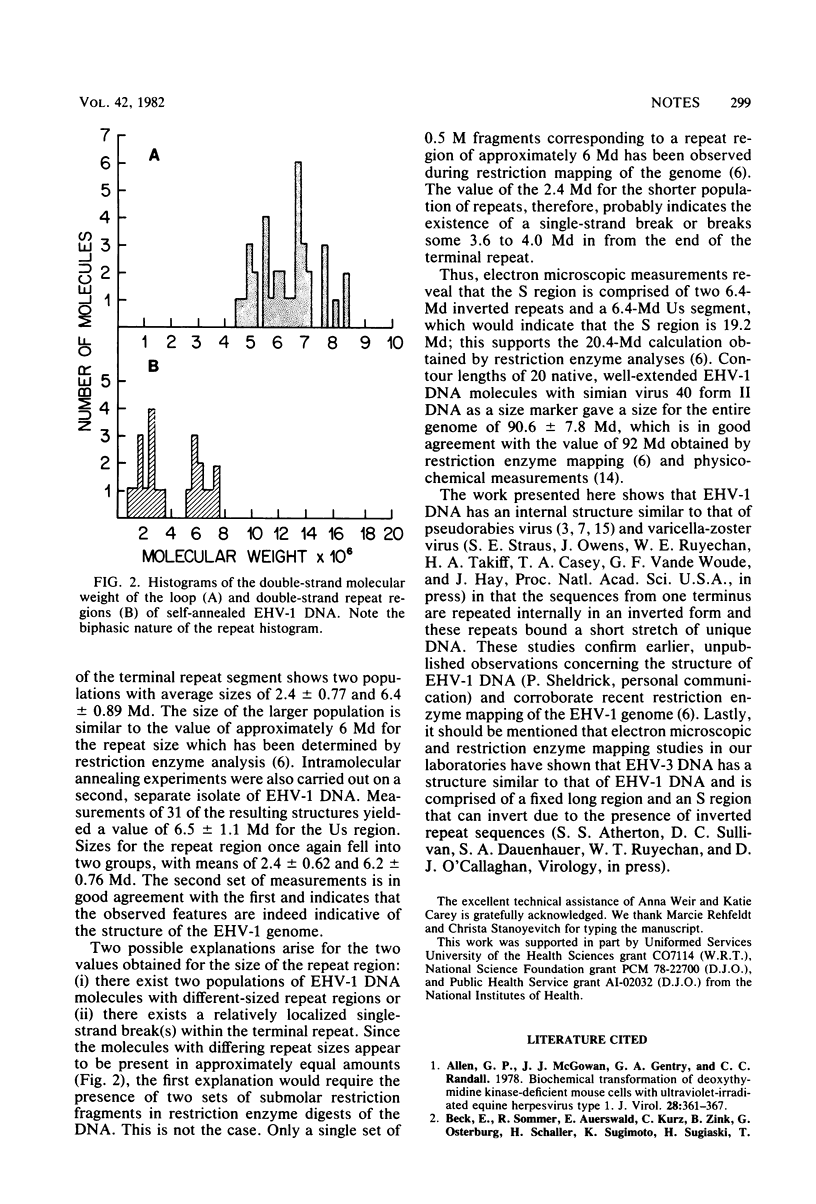
Electron microscopic studies of equine herpesvirus DNA revealed that single strands that were allowed to reanneal formed single-stranded loops with double-stranded stems only at one end of the molecule. These observations support restriction enzyme analyses which indicate that the 92-megadalton DNA molecule exists as a long region of unique sequences covalently linked to a short region. The short region is comprised of an internal unique sequence, which forms the loop during reannealing of single strands, and two terminal inverted repeat sequences that bracket the unique sequence and form the double-stranded stem structure observed upon reannealing of single strands. Measurements of the unique sequence and terminal inverted repeat subgenomic sequences indicate a size of 6.4 megadaltons for each and thus fix the size of the short region at approximately 19.2 megadaltons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., McGowan J. J., Gentry G. A., Randall C. C. Biochemical transformation of deoxythymidine kinase-deficient mouse cells with UV-irradiated equine herpesvirus type 1. J Virol. 1978 Oct;28(1):361–367. doi: 10.1128/jvi.28.1.361-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., Rixon F. J., Blankenship M. L. Analysis of the structure of the genome of pseudorabies virus. Virology. 1979 Jun;95(2):285–294. doi: 10.1016/0042-6822(79)90484-7. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Henry B. E., Robinson R. A., Dauenhauer S. A., Atherton S. S., Hayward G. S., O'Callaghan D. J. Structure of the genome of equine herpesvirus type 1. Virology. 1981 Nov;115(1):97–114. doi: 10.1016/0042-6822(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Jean J. H., Blankenship M. L., Ben-Porat T. Replication of herpesvirus DNA. I. Electron microscopic analysis of replicative structures. Virology. 1977 Jun 15;79(2):281–291. doi: 10.1016/0042-6822(77)90355-5. [DOI] [PubMed] [Google Scholar]
- Robinson R. A., Henry B. E., Duff R. G., O'Callaghan D. J. Oncogenic transformation by equine herpesviruses (EHV). I. Properties of hamster embryo cells transformed by ultraviolet-irradiated EHV-1. Virology. 1980 Mar;101(2):335–362. doi: 10.1016/0042-6822(80)90449-3. [DOI] [PubMed] [Google Scholar]
- Robinson R. A., Tucker P. W., Dauenhauer S. A., O'Callaghan D. J. Molecular cloning of equine herpesvirus type 1 DNA: analysis of standard and defective viral genomes and viral sequences in oncogenically transformed cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6684–6688. doi: 10.1073/pnas.78.11.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. A., Vance R. B., O'Callaghan D. J. Oncogenic transformation by by equine herpesviruses. II. Coestablishment of persistent infection and oncogenic transformation of hamster embryo cells by equine herpesvirus type 1 preparations enriched for defective interfering particles. J Virol. 1980 Oct;36(1):204–219. doi: 10.1128/jvi.36.1.204-219.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOEHNER R. L., GENTRY G. A., RANDALL C. C. SOME PHYSICOCHEMICAL CHARACTERISTICS OF EQUINE ABORTION VIRUS NUCLEIC ACID. Virology. 1965 Jul;26:394–405. doi: 10.1016/0042-6822(65)90003-6. [DOI] [PubMed] [Google Scholar]
- Stevely W. S. Inverted repetition in the chromosome of pseudorabies virus. J Virol. 1977 Apr;22(1):232–234. doi: 10.1128/jvi.22.1.232-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




